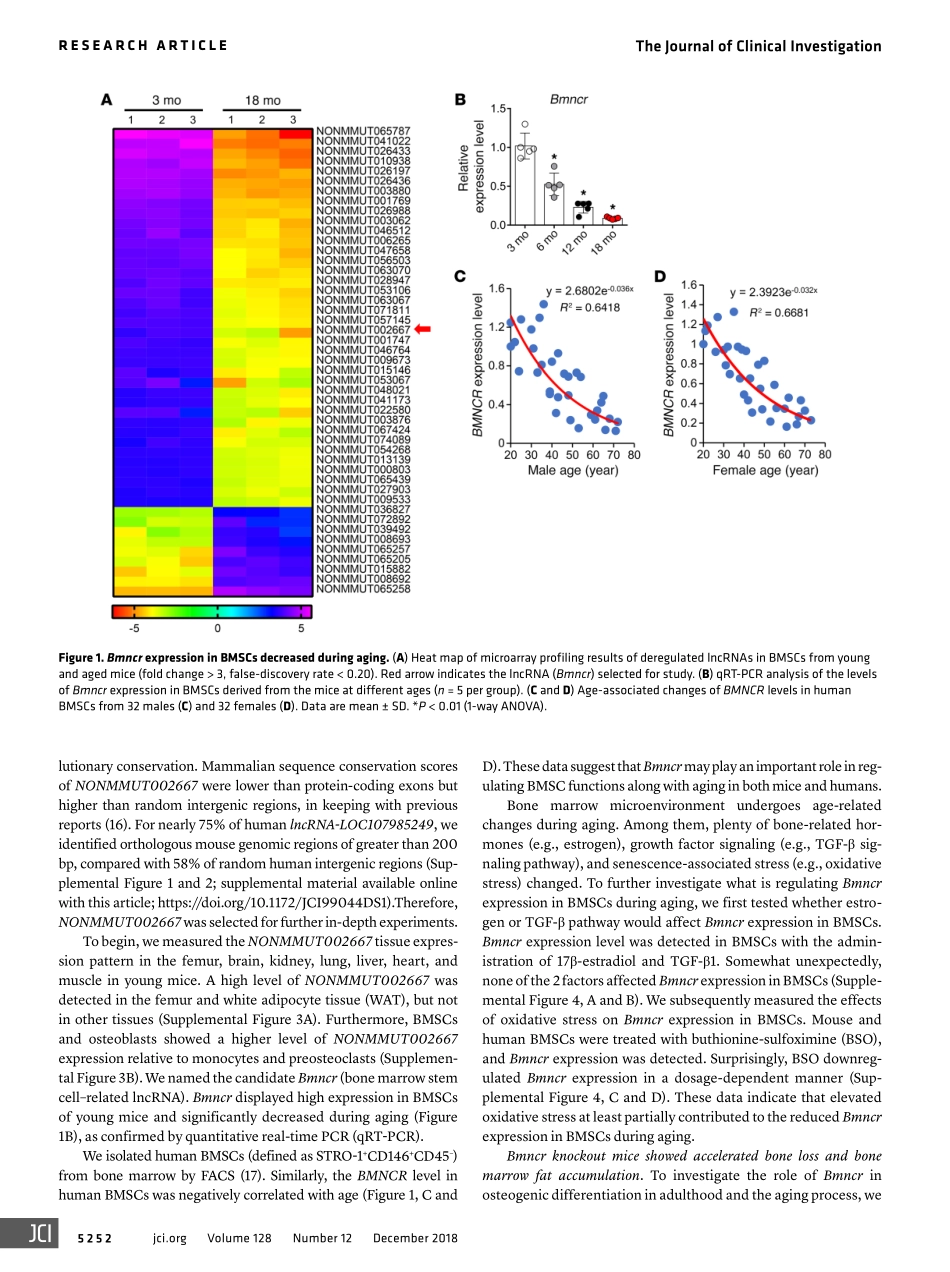
LongnoncodingRNABmncrregulatesmesenchymalstemcellfateduringskeletalagingChang-JunLi,…,YanHuang,Xiang-HangLuoJClinInvest.2018;128(12):5251-5266.https://doi.org/10.1172/JCI99044.Bonemarrowmesenchymalstemcells(BMSCs)exhibitanage-relatedlineageswitchbetweenosteogenicandadipogenicfates,whichcontributestobonelossandadiposity.HereweidentifiedalongnoncodingRNA,Bmncr,whichregulatedthefateofBMSCsduringaging.MicedepletedofBmncr(Bmncr-KO)showeddecreasedbonemassandincreasedbonemarrowadiposity,whereastransgenicoverexpressionofBmncr(Bmncr-Tg)alleviatedbonelossandbonemarrowfataccumulation.BmncrregulatedtheosteogenicnicheofBMSCsbymaintainingextracellularmatrixproteinfibromodulin(FMOD)andactivationoftheBMP2pathway.Bmncraffectedlocal3DchromatinstructureandtranscriptionofFmod.TheabsenceofFmodmodifiedthebonephenotypeofBmncr-Tgmice.FurtheranalysisrevealedthatBmncrwouldserveasascaffoldtofacilitatetheinteractionofTAZandABL,andthusfacilitatetheassemblyoftheTAZandRUNX2/PPARGtranscriptionalcomplex,promotingosteogenesisandinhibitingadipogenesis.Adeno-associatedviral-mediatedoverexpressionofTazinosteoprogenitorsalleviatedbonelossandmarrowfataccumulationinBmncr-KOmice.Furthermore,restoringBMNCRlevelsinhumanBMSCsreversedtheage-relatedswitchbetweenosteoblastandadipocytedifferentiation.OurfindingsindicatethatBmncrisakeyregulatoroftheage-relatedosteogenicnichealterationandcellfateswitchofBMSCs.ResearchArticleEndocrinologyFindthelatestversion:http://jci.me/99044/pdfTheJournalofClinicalInvestigationRESEARCHARTICLE5251jci.orgVolume128Number12December2018IntroductionGuidedbygeneticandmolecularmediators,aswellasthelocalmicroenvironment,bonemarrowmesenchymalstemcells(BMSCs)differentiateintovariousmaturecelltypes,includingadipocytesandosteoblasts(1–3).Age-relatedosteoporosisischaracterizedbyreducedboneformationandtheaccumulationoffatinthebonemarrowcompartment.Withage,BMSCsaremoreinclinedtounder-godifferentiationintoadipocytesratherthanosteoblasts,resultinginanincreasednumberofadipocytesandadecreasednu...