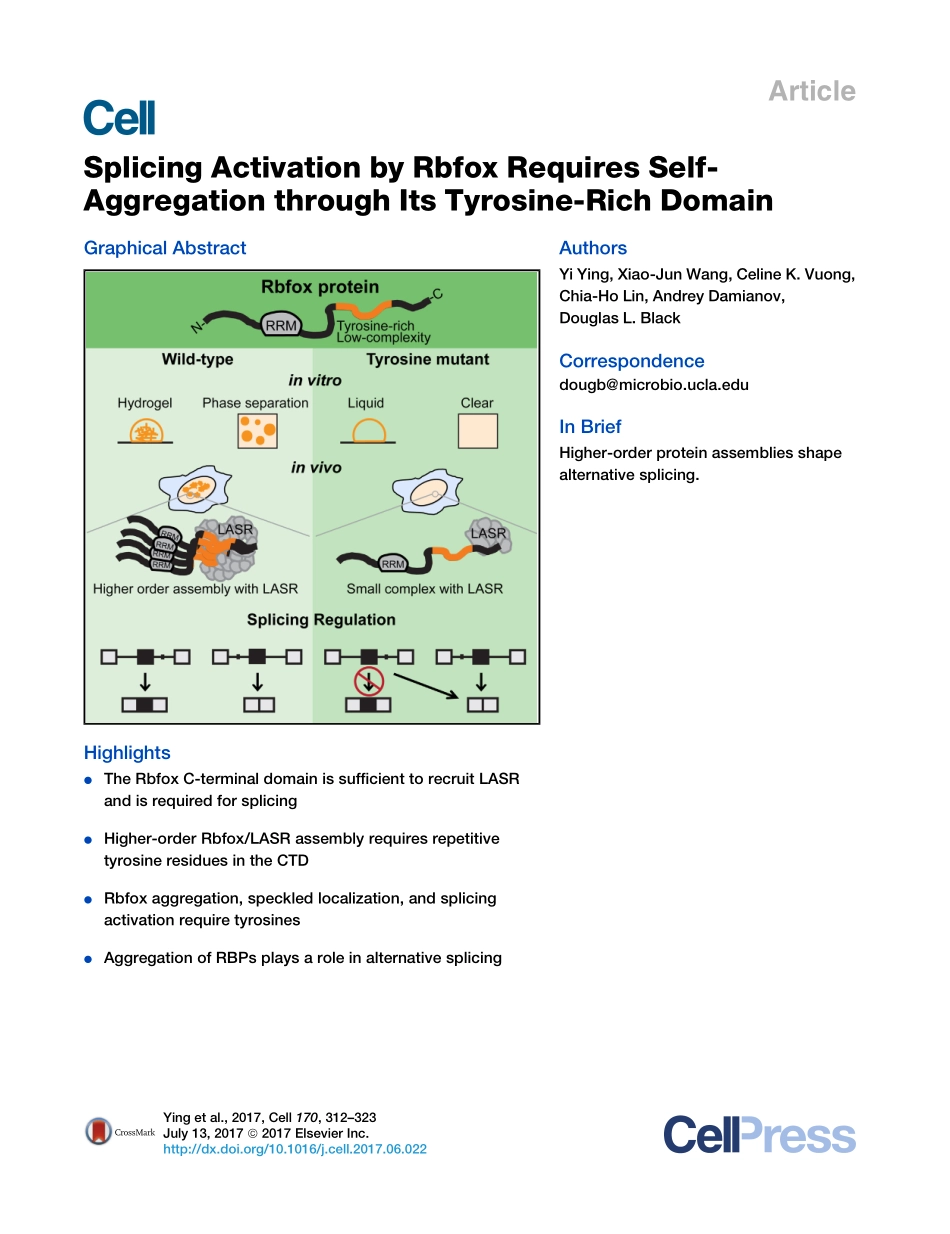
ArticleSplicingActivationbyRbfoxRequiresSelf-AggregationthroughItsTyrosine-RichDomainGraphicalAbstractHighlightsdTheRbfoxC-terminaldomainissufficienttorecruitLASRandisrequiredforsplicingdHigher-orderRbfox/LASRassemblyrequiresrepetitivetyrosineresiduesintheCTDdRbfoxaggregation,speckledlocalization,andsplicingactivationrequiretyrosinesdAggregationofRBPsplaysaroleinalternativesplicingAuthorsYiYing,Xiao-JunWang,CelineK.Vuong,Chia-HoLin,AndreyDamianov,DouglasL.BlackCorrespondencedougb@microbio.ucla.eduInBriefHigher-orderproteinassembliesshapealternativesplicing.Yingetal.,2017,Cell170,312–323July13,2017ª2017ElsevierInc.http://dx.doi.org/10.1016/j.cell.2017.06.022ArticleSplicingActivationbyRbfoxRequiresSelf-AggregationthroughItsTyrosine-RichDomainYiYing,1,3Xiao-JunWang,2CelineK.Vuong,1,3Chia-HoLin,2AndreyDamianov,2andDouglasL.Black2,3,4,*1MolecularBiologyInterdepartmentalDoctoralProgram2DepartmentofMicrobiology,Immunology,andMolecularGenetics3MolecularBiologyInstituteUniversityofCalifornia,LosAngeles,LosAngeles,CA,USA4LeadContact*Correspondence:dougb@microbio.ucla.eduhttp://dx.doi.org/10.1016/j.cell.2017.06.022SUMMARYProteinsoftheRbfoxfamilyactwithacomplexofproteinscalledtheLargeAssemblyofSplicingRegulators(LASR).WefindthatRbfoxinteractswithLASRviaitsC-terminaldomain(CTD),andthisdomainisessentialforitssplicingactivity.InadditiontoLASRrecruitment,alow-complexity(LC)sequencewithintheCTDcontainsrepeatedtyrosinesthatmediatehigher-orderassemblyofRbfox/LASRandarerequiredforsplicingactivationbyRbfox.Thissequencespontaneouslyaggregatesinsolutiontoformfibrousstructuresandhydrogels,suggestinganassemblysimilartotheinsolublecellularinclusionsformedbyFUSandotherproteinsinneurologicdisease.Unlikethepathologicalaggre-gates,wefindthatassemblyoftheRbfoxCTDplaysanessentialroleinitsnormalsplicingfunction.Ratherthansimplerecruitmentofindividualregula-torstoatargetexon,alternativesplicingchoicesalsodependonthehigher-orderassemblyoftheseregulatorswithinthenucleus.INTRODUCTIONRNA-bindingpro...