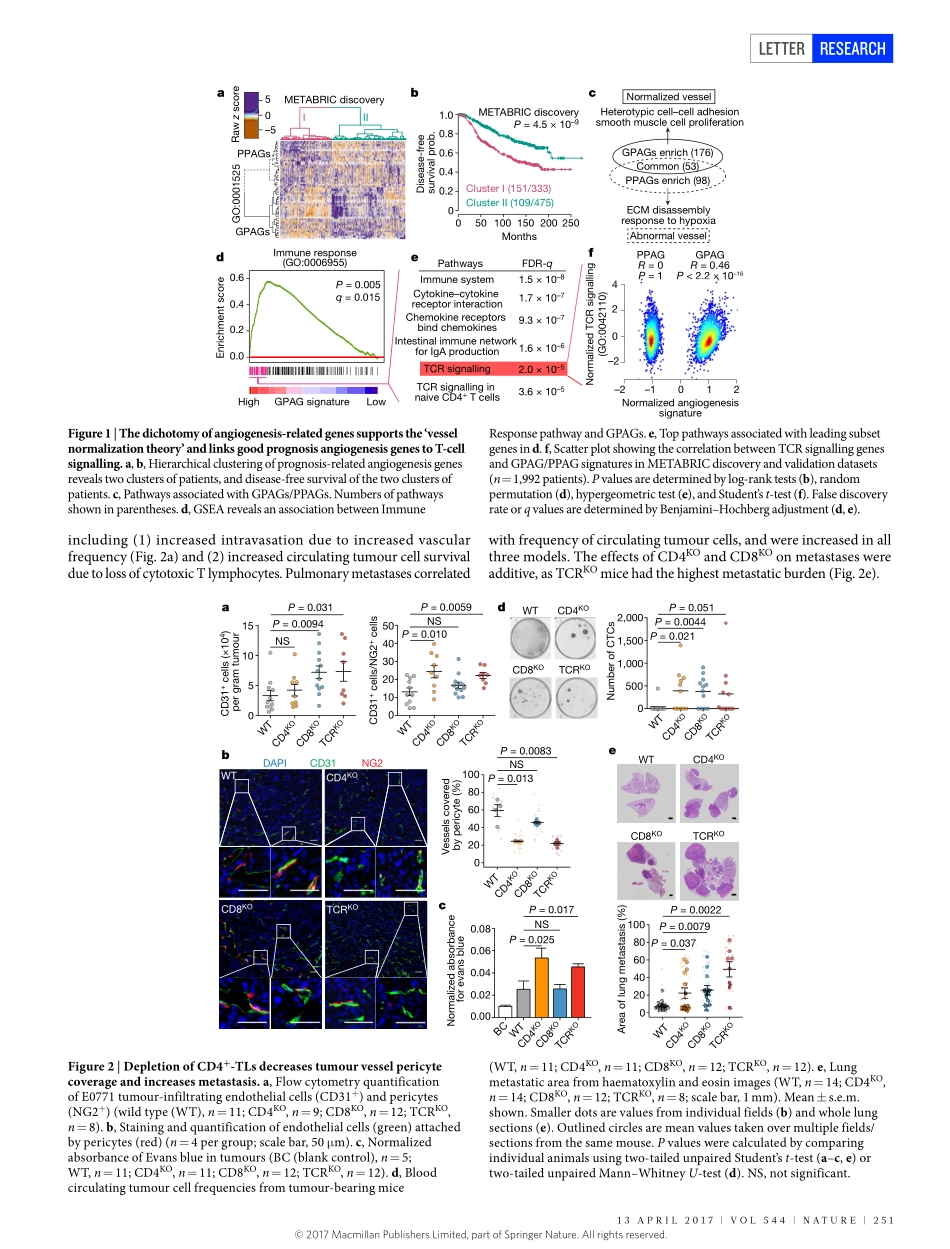
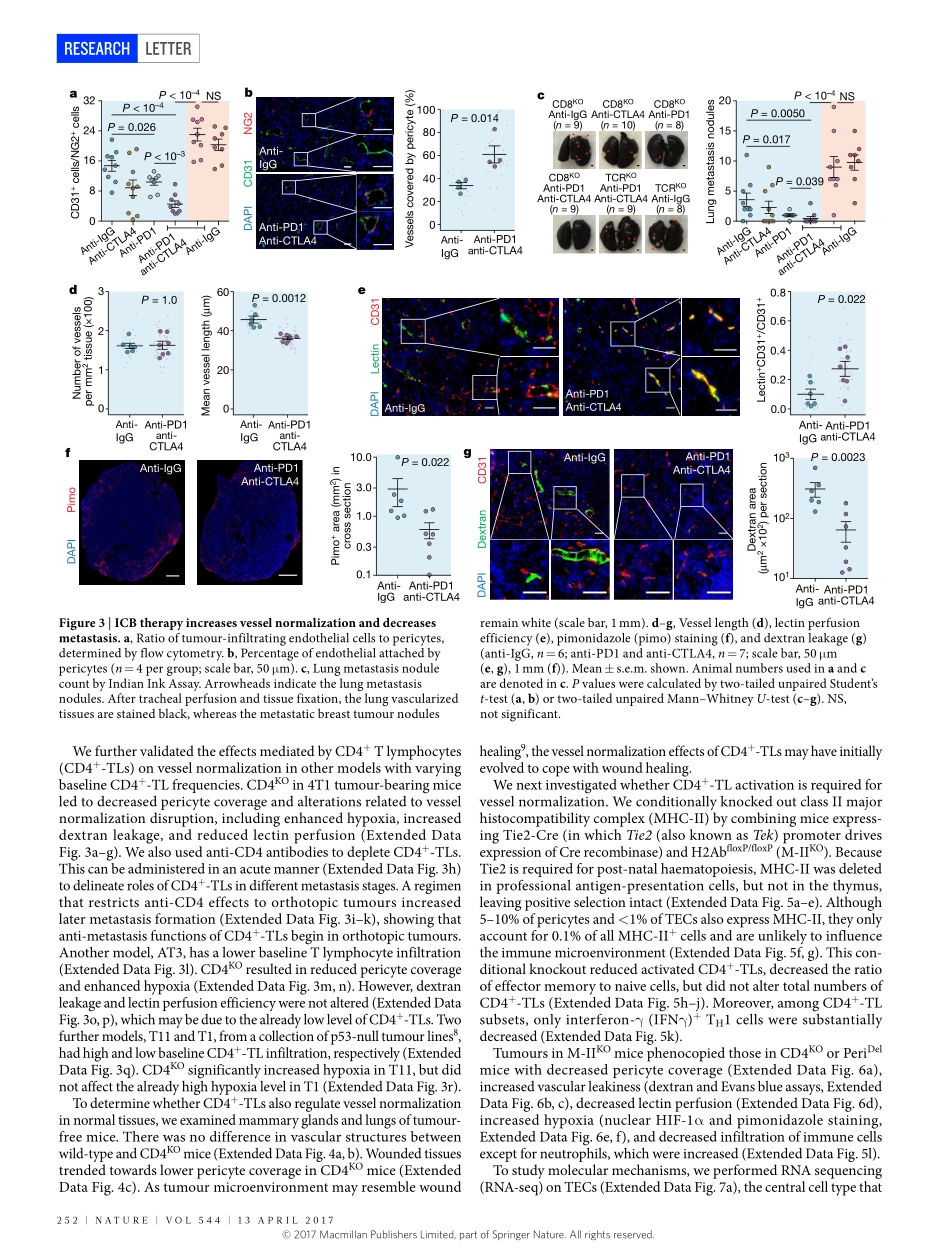
250|NATURE|VOL544|13ApRiL2017LETTERdoi:10.1038/nature21724MutualregulationoftumourvesselnormalizationandimmunostimulatoryreprogrammingLinTian1,2,3,AmitGoldstein1,2,4,HaiWang1,2,4,HinChingLo1,2,5,ikSunKim1,2,5,ThomasWelte1,2,4,KuanweiSheng5,6,LaceyE.Dobrolecki1,XiaomeiZhang1,Nagireddyputluri2,3,4,ThuyL.phung7,SenduraiA.Mani8,FabioStossi2,4,ArunSreekumar2,3,4,MichaelA.Mancini2,4,WilliamK.Decker2,7,9,ChenghangZong2,5,6,10,MichaelT.Lewis1,2,4&XiangH.-F.Zhang1,2,4,10Blockadeofangiogenesiscanretardtumourgrowth,butmayalsoparadoxicallyincreasemetastasis1,2.Thisparadoxmayberesolvedbyvesselnormalization3,whichinvolvesincreasedpericytecoverage,improvedtumourvesselperfusion,reducedvascularpermeability,andconsequentlymitigatedhypoxia3.Althoughtheseprocessesaltertumourprogression,theirregulationispoorlyunderstood.Hereweshowthattype1Thelper(TH1)cellsplayacrucialroleinvesselnormalization.Bioinformaticanalysesrevealedthatgeneexpressionfeaturesrelatedtovesselnormalizationcorrelatewithimmunostimulatorypathways,especiallyTlymphocyteinfiltrationoractivity.Todelineatethecausalrelationship,weusedvariousmousemodelswithvesselnormalizationorTlymphocytedeficiencies.AlthoughdisruptionofvesselnormalizationreducedTlymphocyteinfiltrationasexpected4,reciprocaldepletionorinactivationofCD4+Tlymphocytesdecreasedvesselnormalization,indicatingamutuallyregulatoryloop.Inaddition,activationofCD4+Tlymphocytesbyimmunecheckpointblockadeincreasedvesselnormalization.TH1cellsthatsecreteinterferon-γareamajorpopulationofcellsassociatedwithvesselnormalization.Patient-derivedxenografttumoursgrowinginimmunodeficientmiceexhibitedenhancedhypoxiacomparedtotheoriginaltumoursinimmunocompetenthumans,andhypoxiawasreducedbyadoptiveTH1transfer.OurfindingselucidateanunexpectedroleofTH1cellsinvasculatureandimmunereprogramming.TH1cellsmaybeamarkerandadeterminantofbothimmunecheckpointblockadeandanti-angiogenesisefficacy.Tobetterunderstandangiogenesis,weexaminedangiogenesis-relatedgenesinbreastcancerusingtheMETABRICdatabase5....