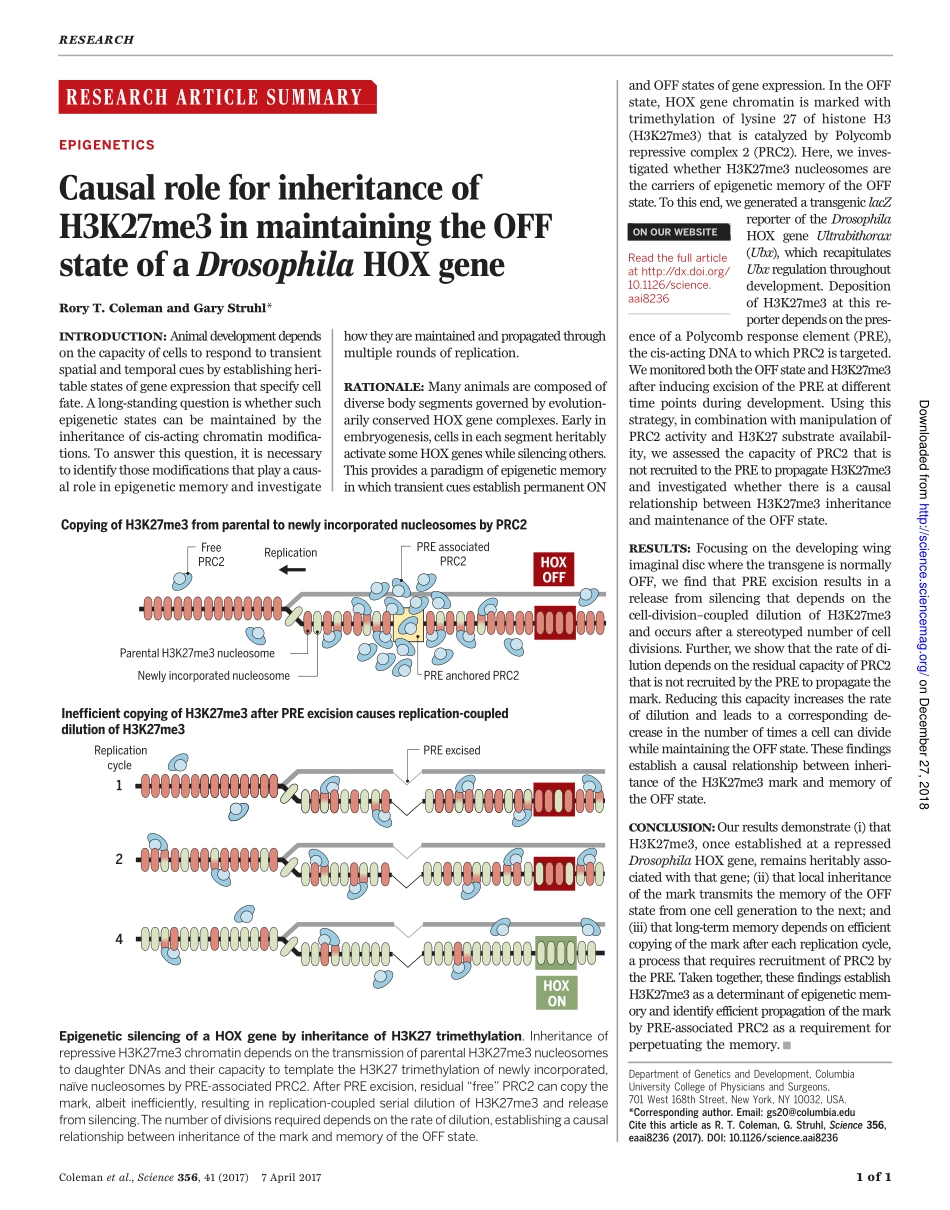
RESEARCHARTICLESUMMARY◥EPIGENETICSCausalroleforinheritanceofH3K27me3inmaintainingtheOFFstateofaDrosophilaHOXgeneRoryT.ColemanandGaryStruhl*INTRODUCTION:Animaldevelopmentdependsonthecapacityofcellstorespondtotransientspatialandtemporalcuesbyestablishingheri-tablestatesofgeneexpressionthatspecifycellfate.Along-standingquestioniswhethersuchepigeneticstatescanbemaintainedbytheinheritanceofcis-actingchromatinmodifica-tions.Toanswerthisquestion,itisnecessarytoidentifythosemodificationsthatplayacaus-alroleinepigeneticmemoryandinvestigatehowtheyaremaintainedandpropagatedthroughmultipleroundsofreplication.RATIONALE:Manyanimalsarecomposedofdiversebodysegmentsgovernedbyevolution-arilyconservedHOXgenecomplexes.Earlyinembryogenesis,cellsineachsegmentheritablyactivatesomeHOXgeneswhilesilencingothers.ThisprovidesaparadigmofepigeneticmemoryinwhichtransientcuesestablishpermanentONandOFFstatesofgeneexpression.IntheOFFstate,HOXgenechromatinismarkedwithtrimethylationoflysine27ofhistoneH3(H3K27me3)thatiscatalyzedbyPolycombrepressivecomplex2(PRC2).Here,weinves-tigatedwhetherH3K27me3nucleosomesarethecarriersofepigeneticmemoryoftheOFFstate.Tothisend,wegeneratedatransgeniclacZreporteroftheDrosophilaHOXgeneUltrabithorax(Ubx),whichrecapitulatesUbxregulationthroughoutdevelopment.DepositionofH3K27me3atthisre-porterdependsonthepres-enceofaPolycombresponseelement(PRE),thecis-actingDNAtowhichPRC2istargeted.WemonitoredboththeOFFstateandH3K27me3afterinducingexcisionofthePREatdifferenttimepointsduringdevelopment.Usingthisstrategy,incombinationwithmanipulationofPRC2activityandH3K27substrateavailabil-ity,weassessedthecapacityofPRC2thatisnotrecruitedtothePREtopropagateH3K27me3andinvestigatedwhetherthereisacausalrelationshipbetweenH3K27me3inheritanceandmaintenanceoftheOFFstate.RESULTS:FocusingonthedevelopingwingimaginaldiscwherethetransgeneisnormallyOFF,wefindthatPREexcisionresultsinareleasefromsilencingthatdependsonthecell-division–coupleddilutionofH3K27me3andoccursafterastereotypednumberofcelldivi...