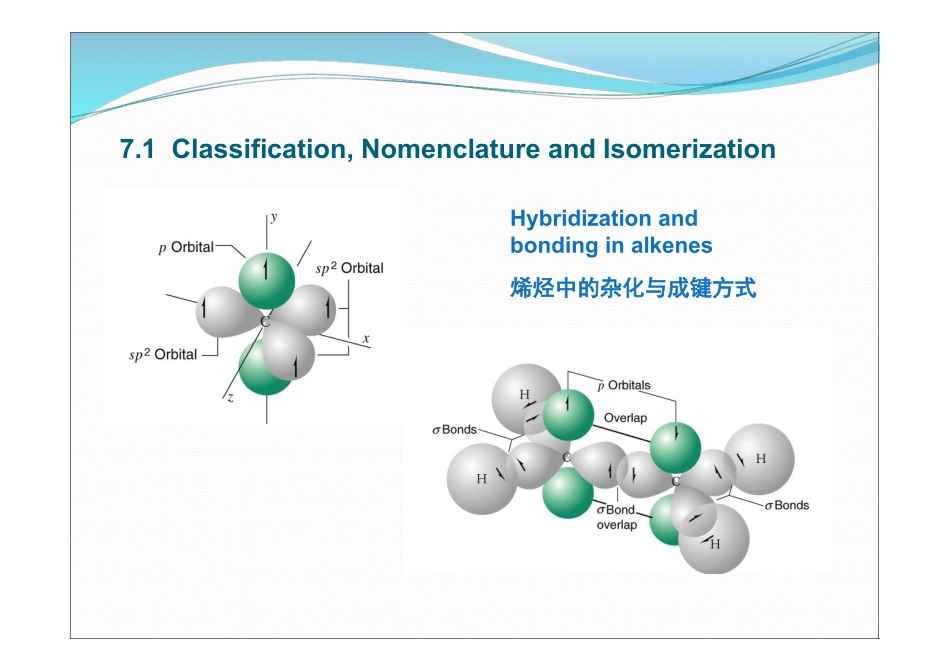
Alkenes第七章:烯烃OrganicChemistryA(1)ByProf.LiYan-MeiTsinghuaUniversityContentContentContent7.1Classification,NomenclatureandIsomerization7.2SpectrumData&PhysicalProperties7.3ChemicalReactions7.4PreparationofAlkenesHybridizationandbondinginalkenes烯烃中的杂化与成键方式7.1Classification,NomenclatureandIsomerizationciscis--trans-trans-半扭曲型过渡态(E)-3-ethyl-6-methyloct-4-eneA>DandB>CA>DandC>BZformEform(5R,2E)-5-(5R,2E)-5-甲基甲基-3--3-丙基丙基-2--2-庚烯庚烯CCH3CHCH2CH2CH3CH2CCH2CH3CH3H7.2.1SpectrumData7.2.2PhysicalProperties7.2SpectrumData&PhysicalProperties7.2.1SpectrumData11H-NMRH-NMR5.25.24.5-6.54.5-6.51.71.71313C-NMRC-NMR=C120IRIRC=Cstretching1680-1620cm-1C-Hstretching3100-3010cm-1••共轭体系:共轭体系:CC==CC伸缩振动移向低波数伸缩振动移向低波数••端烯:端烯:••顺顺//反:反:985-885cm985-885cm-1-1980-965cm980-965cm-1-1730-650cm730-650cm-1-17.2.2PhysicalData==0.35D0.35D==0.35-0.37D0.35-0.37DWhichhashigherboilingpoint?Whichhashigherboilingpoint?7.3Chemicalreactions7.3.1Hydrogenation7.3.2Electrophilicaddition7.3.3FreeRadicalAddition7.3.4Oxidation7.3.5α-Halogenation7.3.6ReactionswithCarbene7.3.7Isomerization7.3.8PolymerizationCatalytichydration““烯烯””,缺氢,可加,缺氢,可加HH22p-pp-p键,不稳定,可极化能力强-反应原因键,不稳定,可极化能力强-反应原因与缺电子或带正电荷的分子反应(亲电)-反应动力与缺电子或带正电荷的分子反应(亲电)-反应动力亲电加成亲电加成(Electrophilicaddition)-反应结果反应结果双键上电子云密度大,易给出电子,被氧化双键上电子云密度大,易给出电子,被氧化Oxidation双键共轭稳定碳负离子双键共轭稳定碳负离子具有部分酸性具有部分酸性Acidityp-conjugationCH2+烯丙位7.3.1Hydrogenation催化加氢Cat.:HomogenouscatalystCat.:Homogenouscatalyst((均相催化剂均相催化剂))::egeg::[(C[(C66HH55))33P]P]33RhClRhClWilkisonWilkisonCatalystCatalystHeterogenousHeterogenouscatalystcatalyst((异相催化剂异相催化剂))::egeg:Pt,Pd/CaCO:Pt,Pd/CaCO33,BaSO,BaSO44,Al,Al22OO33egeg:RaneyNi::RaneyNi:NiAlNiAl++NaOHNaOH...