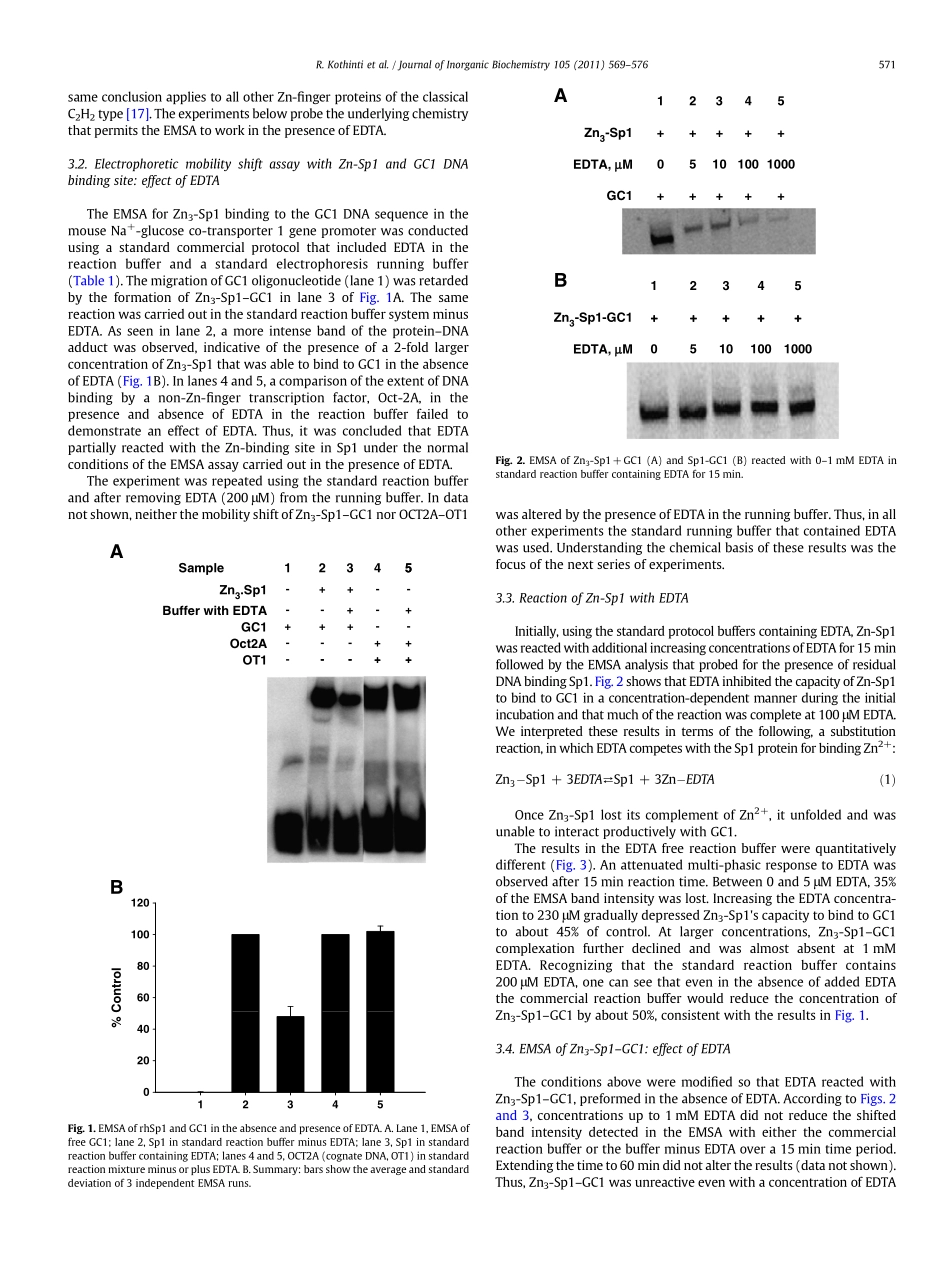
Electrophoreticmobilityshiftassayofzincfingerproteins:CompetitionforZn2+boundtoSp1inprotocolsincludingEDTARajendraKothintia,NiloofarM.Tabatabaib,DavidH.Peteringa,⁎aDepartmentofChemistryandBiochemistry,UniversityofWisconsin–Milwaukee,Milwaukee,WI53201-0413,USAbDivisionofEndocrinology,MetabolismandClinicalNutritionandKidneyDiseaseCenter,MedicalCollegeofWisconsin,Wauwatosa,WI53226,USAabstractarticleinfoArticlehistory:Received25January2010Receivedinrevisedform18August2010Accepted19August2010Availableonline31August2010Keywords:ZincfingerProteinEDTAEMSATheelectrophoreticmobilityshiftassay(EMSA)offersaprincipalmethodtodetectspecificDNA–proteininteractions.Ascommonlyconducted,thereactionandelectrophoresisrunningbufferscontainlargeconcentrationsofEDTA.EDTAhaslargeaffinityforZn2+andreadilycompeteswithzincfingerpeptidesforZn2+resultinginproteinunfolding.Nevertheless,EMSAisroutinelyusedtodetectzincfingerprotein–DNAadducts.Thispaperexaminesthechemistrythatpermitsthedetectionofzincfinger–DNAcomplexesinthepresenceofEDTA,usingZn3-Sp1andacognateDNAbindingsite,GC1.TwiceasmuchadductwasdetectedwhenthereactionwasconductedintheabsencethaninthepresenceofEDTA.TheobservationofZn-Sp1–GC1wasshowntodependonthreeproperties:theinertnessofZn-Sp1–GC1toreactionwithEDTAandthecomparativelysimilarratesofreactionofEDTAandGC1withZn3-Sp1undertheconditionsoftheassaythatpermitsomeZn3-Sp1–GC1toform.InquiringaboutthemechanismofstabilizationofZn3-Sp1byGC1,EDTAreadilyreactedwithZn3-Sp1boundtoanon-specificDNA,(polydI-dC).Twostructurallysimilarbutoppositelychargedchelators,nitrilotriacetate(NTA)andtris-(2-ethylaminoethyl)amine(TREN),thatreactwithfreeZn3-Sp1failedtocompeteforzincboundintheZn3-Sp1–GC-1adduct.Onthebasisofthese,otherresultsindicatedthatthestabilityofZn3-Sp1–GC-1hasathermodynamic,notakineticorigin.ItisconcludedthattheobservationofzincfingerproteinsintheEMSArestsonafortuitoussetofchemicalpropertiesthatmayvarydependingonthestructuresinvolved.©2010ElsevierInc.Allrightsreserved.1.Introducti...