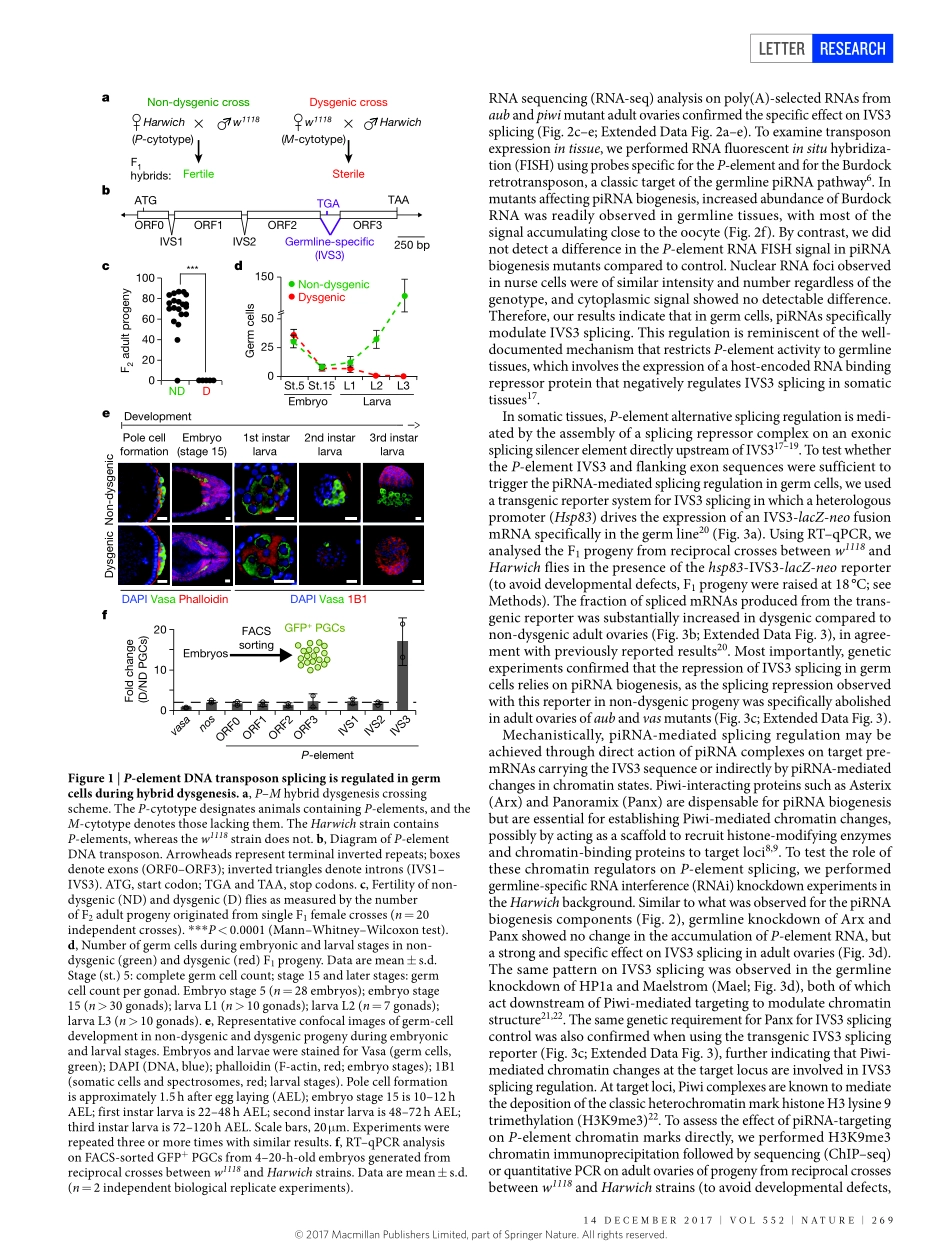
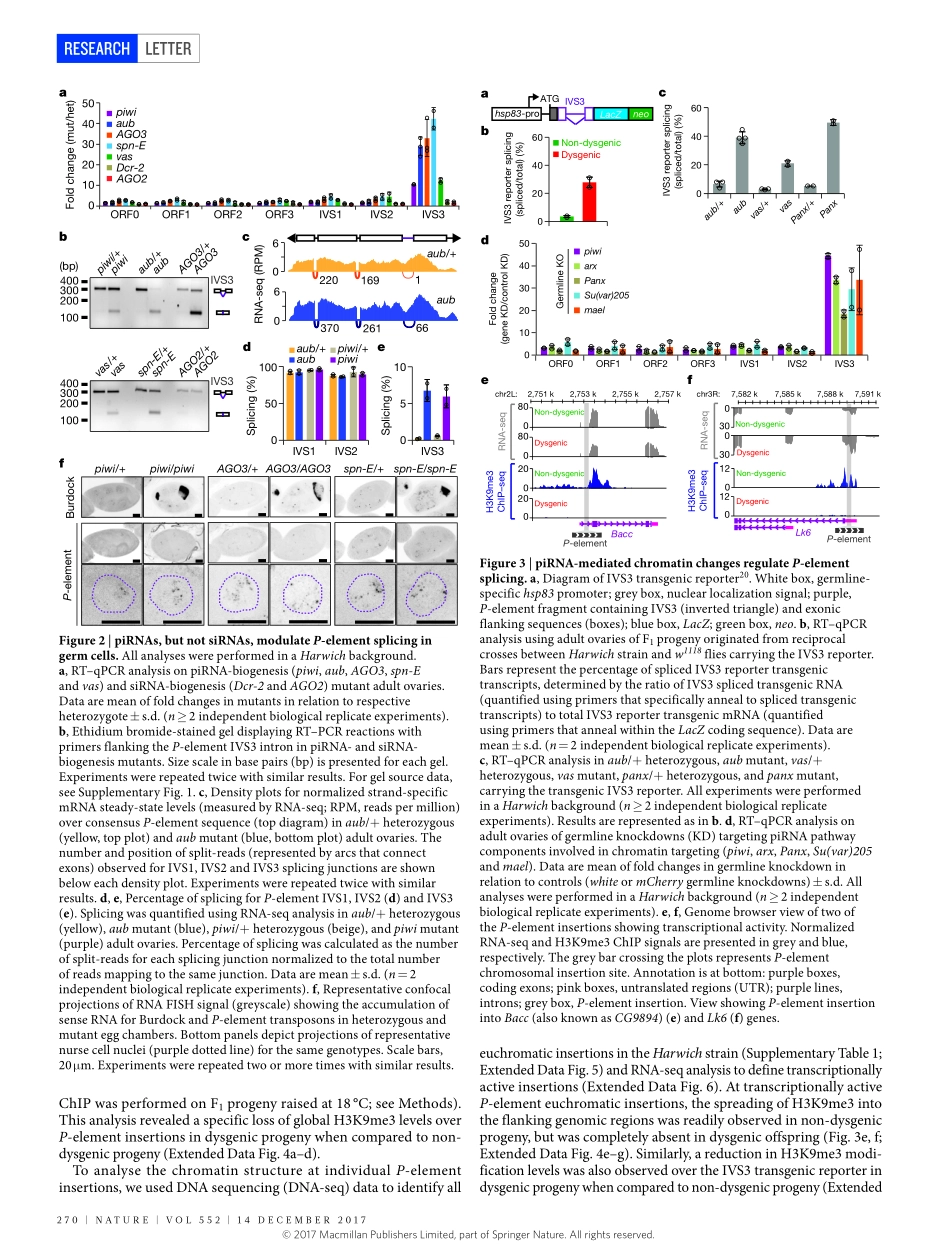
268|NATURE|VOL552|14dEcEmbER2017LETTERdoi:10.1038/nature25018piRNA-mediatedregulationoftransposonalternativesplicinginthesomaandgermlineFelipeKaramTeixeira1†,martynaOkuniewska1,colind.malone1†,Rémi-Xaviercoux1,donaldc.Rio2&RuthLehmann1Transposableelementscandrivegenomeevolution,buttheirenhancedactivityisdetrimentaltothehostandthereforemustbetightlyregulated1.ThePiwi-interactingsmallRNA(piRNA)pathwayisvitalfortheregulationoftransposableelements,byinducingtranscriptionalsilencingorpost-transcriptionaldecayofmRNAs2.HereweshowthatpiRNAsandpiRNAbiogenesiscomponentsregulateprecursormRNAsplicingofP-transposableelementtranscriptsinvivo,leadingtotheproductionofthenon-transposase-encodingmaturemRNAisoforminDrosophilagermcells.Unexpectedly,weshowthatthepiRNApathwaycomponentsdonotacttoreducetranscriptlevelsoftheP-elementtransposonduringP–Mhybriddysgenesis,asyndromethataffectsgermlinedevelopmentinDrosophila3,4.Instead,splicingregulationismechanisticallyachievedtogetherwithpiRNA-mediatedchangestorepressivechromatinstates,andreliesonthefunctionofthePiwi–piRNAcomplexproteinsAsterix(alsoknownasGtsf1)5–7andPanoramix(Silencio)8,9,aswellasHeterochromatinprotein1a(HP1a;encodedbySu(var)205).Furthermore,weshowthatthismachinery,togetherwiththepiRNAFlamencocluster10,notonlycontrolstheaccumulationofGypsyretrotransposontranscripts11butalsoregulatesthesplicingofGypsymRNAsinculturedovariansomaticcells,aprocessrequiredfortheproductionofinfectiousparticlesthatcanleadtoheritabletranspositionevents12,13.OurfindingsidentifysplicingregulationasanewroleandessentialfunctionforthePiwipathwayinprotectingthegenomeagainsttransposonmobility,andprovideamodelsystemforstudyingtheroleofchromatinstructureinmodulatingalternativesplicingduringdevelopment.Hybriddysgenesisisasyndromethataffectsprogenyinanon-reciprocalfashion,beingnormallyrestrictedtotheoffspringofcrossesinwhichmalescarrytransposableelementsbutwhichfemaleslack3,14(Fig.1a).InDrosophila,thedysgenictraitstriggeredbytheP-elementDNAtransposon4(Fi...