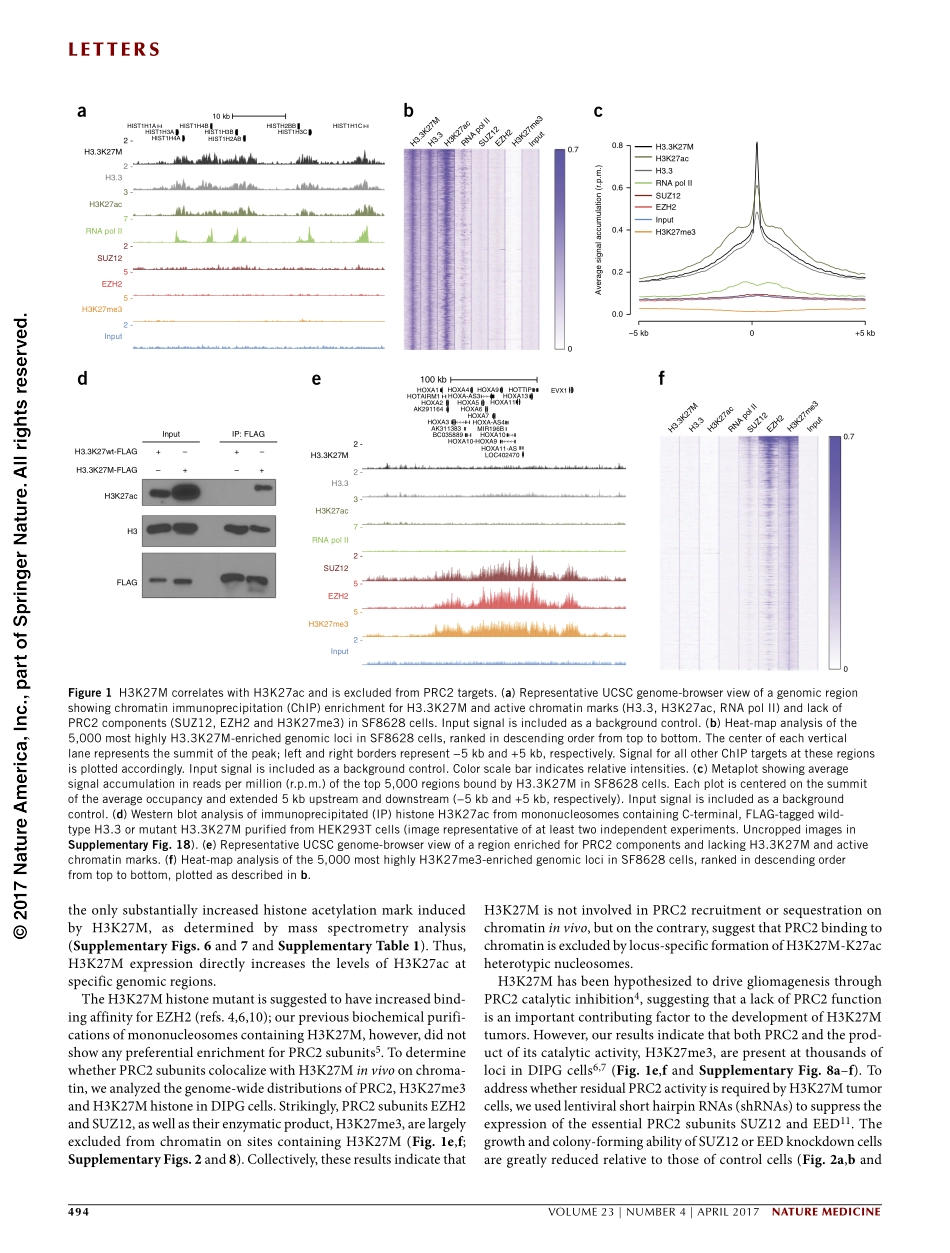
naturemedicineVOLUME23|NUMBER4|APRIL2017493letterSDiffuseintrinsicpontineglioma(DIPG)isahighlyaggressivepediatricbrainstemtumorcharacterizedbyrapidanduniformpatientdemise1.AheterozygouspointmutationofhistoneH3occursinmorethan80%ofthesetumorsandresultsinalysine-to-methioninesubstitution(H3K27M)2,3.Expressionofthishistonemutantisaccompaniedbyareductioninthelevelsofpolycombrepressivecomplex2(PRC2)-mediatedH3K27trimethylation(H3K27me3),andthisishypothesizedtobeadrivingeventofDIPGoncogenesis4,5.DespiteamajorlossofH3K27me3,PRC2activityisstilldetectedinDIPGcellspositiveforH3K27M6,7.ToinvestigatethefunctionalrolesofH3K27MandPRC2inDIPGpathogenesis,weprofiledtheepigenomeofH3K27M-mutantDIPGcellsandfoundthatH3K27MassociateswithincreasedH3K27acetylation(H3K27ac).Inaccordancewithpreviousbiochemicaldata5,themajorityoftheheterotypicH3K27M-K27acnucleosomescolocalizewithbromodomainproteinsatthelociofactivelytranscribedgenes,whereasPRC2isexcludedfromtheseregions;thissuggeststhatH3K27MdoesnotsequesterPRC2onchromatin.ResidualPRC2activityisrequiredtomaintainDIPGproliferativepotential,byrepressingneuronaldifferentiationandfunction.Finally,toexaminethetherapeuticpotentialofblockingtherecruitmentofbromodomainproteinsbyheterotypicH3K27M-K27acnucleosomesinDIPGcells,weperformedtreatmentsinvivowithBETbromodomaininhibitorsanddemonstratethattheyefficientlyinhibittumorprogression,thusidentifyingthisclassofcompoundsaspotentialtherapeuticsinDIPG.ToadvanceunderstandingoftheoncogenicmolecularfunctionoftheH3K27Minoncogenesis,wemappedgenome-wideoccupancyofthemutanthistoneinDIPGcelllinesbychromatinimmunoprecipitation(ChIP)-seq,usinganantibodydirectedagainstH3K27M(SupplementaryFig.1).ToconfirmthespecificityoftheH3K27Mantibody,weexpressedwild-typeorH3K27M-mutanthistoneatequivalentlevelsinHCT116cells,ahumancolorectaltumorcellline(SupplementaryFig.1a)andperformedChIP-seq.ChIP-seqsignalwiththeH3K27MantibodywasdetectedexclusivelyinH3K27M-expressingcells,whereasnoenrichmentwasobservedincellsexpressingthewild...