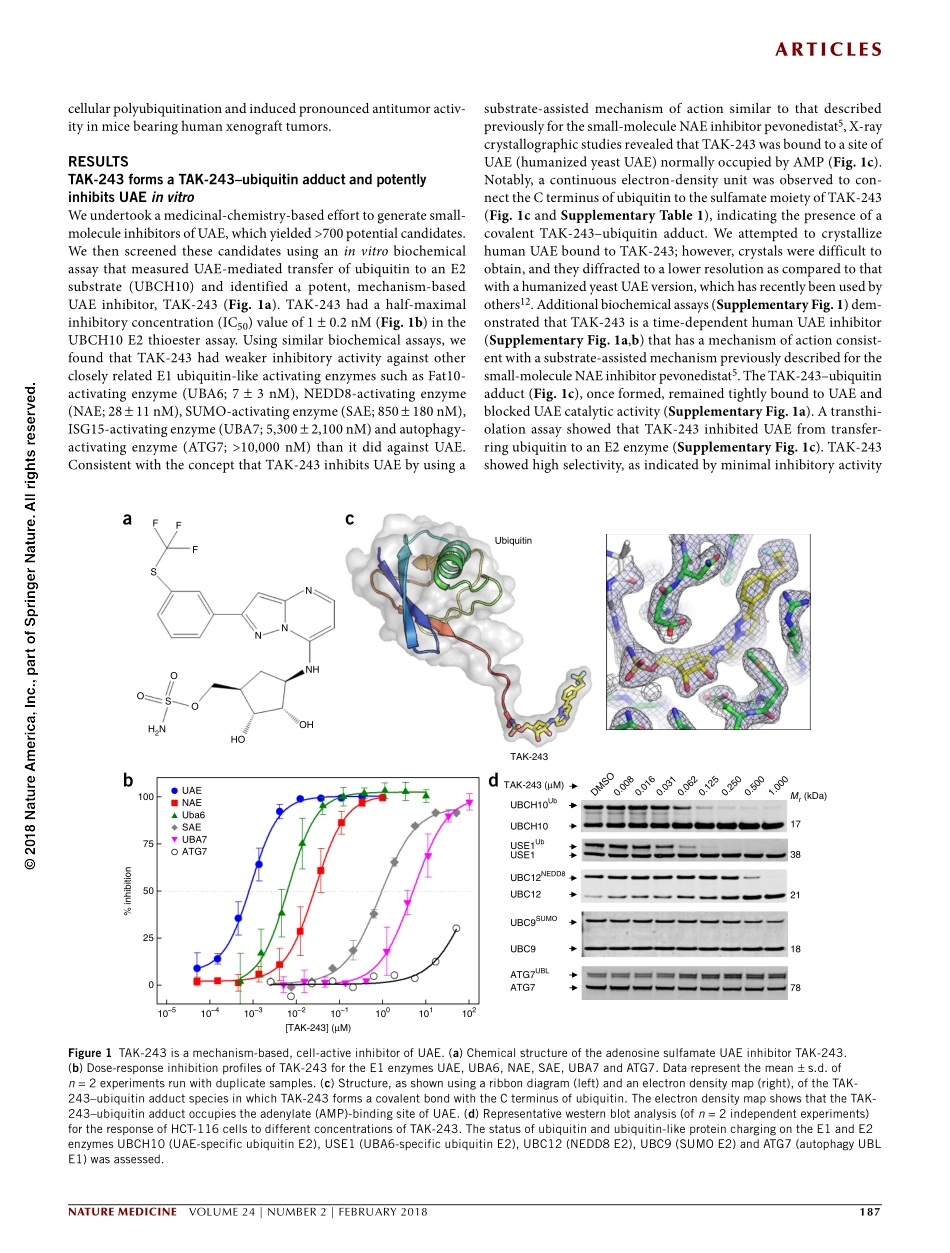
Articles186VOLUME24|NUMBER2|FEBRUARY2018nAturemedicineUbiquitinconjugationinmammalsisinitiatedbytwokeyenzymes,theubiquitinactivatingenzymesUAE(alsoknownasUBE1)andUBA6,whicharecollectivelyreferredtoasE1enzymes.UAE(encodedbytheUBA1gene)isresponsibleforcharginganestimated>99%ofcellularubiquitin,whereasUBA6isresponsibleforcharging<1%ofubiquitin.UAEcatalyzesubiquitin-chargingofall(~35)E2cellularubiquitin-conjugatingenzymes,exceptfortheE2USE1,whosechargingiscatalyzedbyUBA6(ref.1).Ubiquitin-chargedE2enzymescooperatewithcellularE3ligases(forexample,cullin,Ring,HECT,RBRorU-box)todirectspecificcellulartargetproteinubiquitylationmodifications,whichareclassifiedasmonomericorpolyubiquitin;moreover,polyubiquitinchainscanbeoftheLys11(K11),Lys29(K29),Lys48(K48)orLys63(K63)type.Thesevariedubiquitinmodificationsarereadbyubiquitin-bindingproteinsandcandictateoutcomesinwhichthetargetproteinsareeitherdegradedornot.Forexample,K48-linkedpolyubiquitinisassociatedwithproteasome-mediateddegradation,K63-linkedpolyubiquitincanmediateautophagyandsignaltransduction,polyubiquitylationhasbeenshowntobeimportantforsignaltransductionmediatedbythetranscriptionfactorNF-κB2,3,mono-ubiquitylationofhistonescanaltergeneregulation,andmono-ubiquitylationofsurfacereceptorscanmodulatetheirinternalizationandlysosomalproteolysis.Theclinicalsuccessoftheproteasomeinhibitorbortezomib4haspiquedinterestintargetingothercomponentsoftheUPSforcancertherapy.AlthoughthestructuralandmechanisticdiversityofUPSenzymeshaspresentedchallengesforthesmall-moleculeinterrogationofE1,E2,E3anddeubiquitinating(DUB)enzymesincancerbiology,progressisemergingwithongoingclinicalevaluationofpevonedistat(alsoknownasTAK-924andMLN4924;aninhibitoroftheE1fortheubiquitin-likemoleculeNEDD8)5,second-generationIMiDs,inhibitorsoftheE3ligasesforinhibitorofapoptosisprotein(IAP)andmurinedoubleminute2(MDM2),andthevalosin-containingprotein(VCP)inhibitorCB-5083(refs.6–9).UBA1isanessentialgeneinyeast10,11.Althoughnodatahasbeenpublishedontheeffectsofgenet...