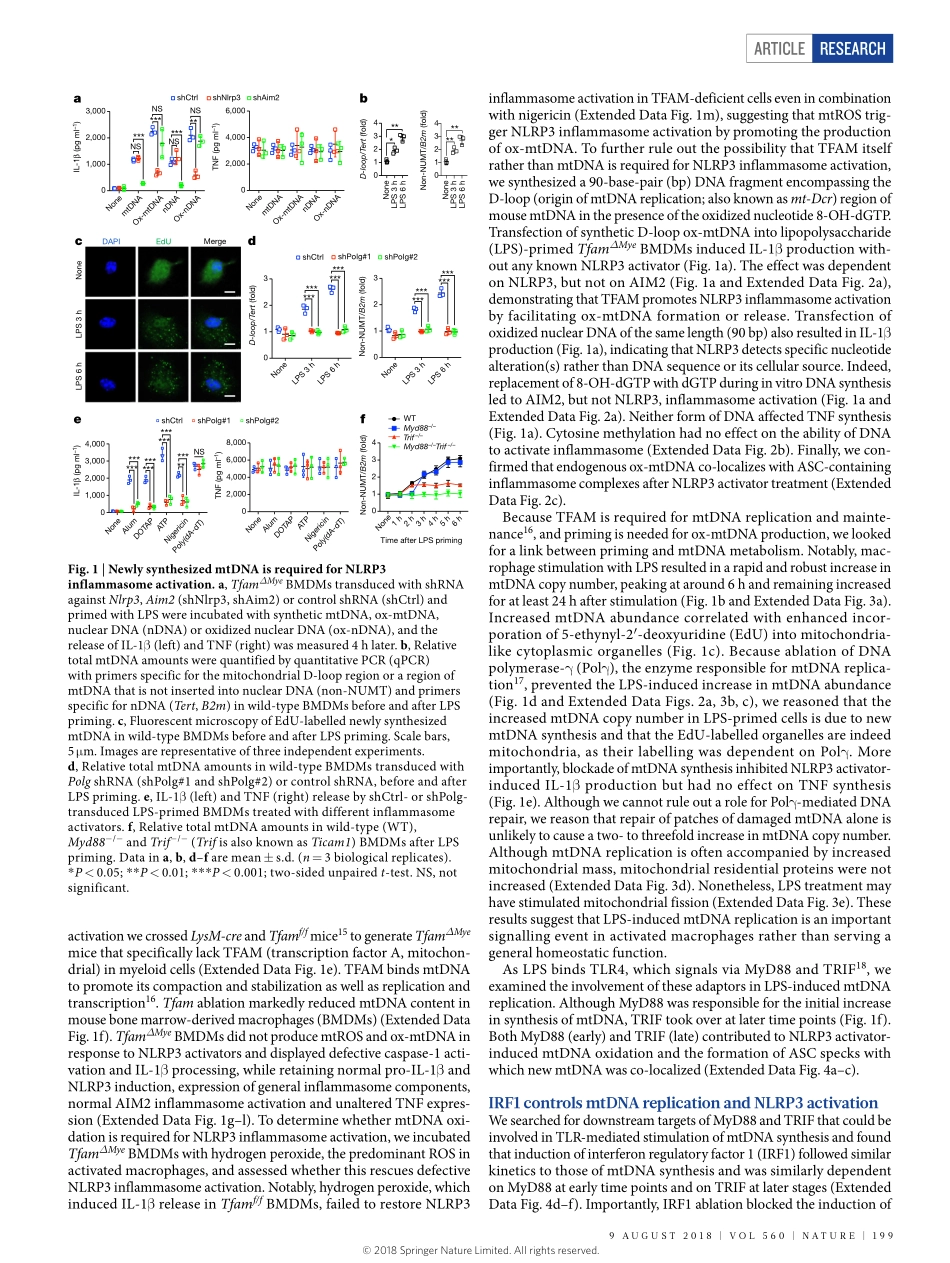
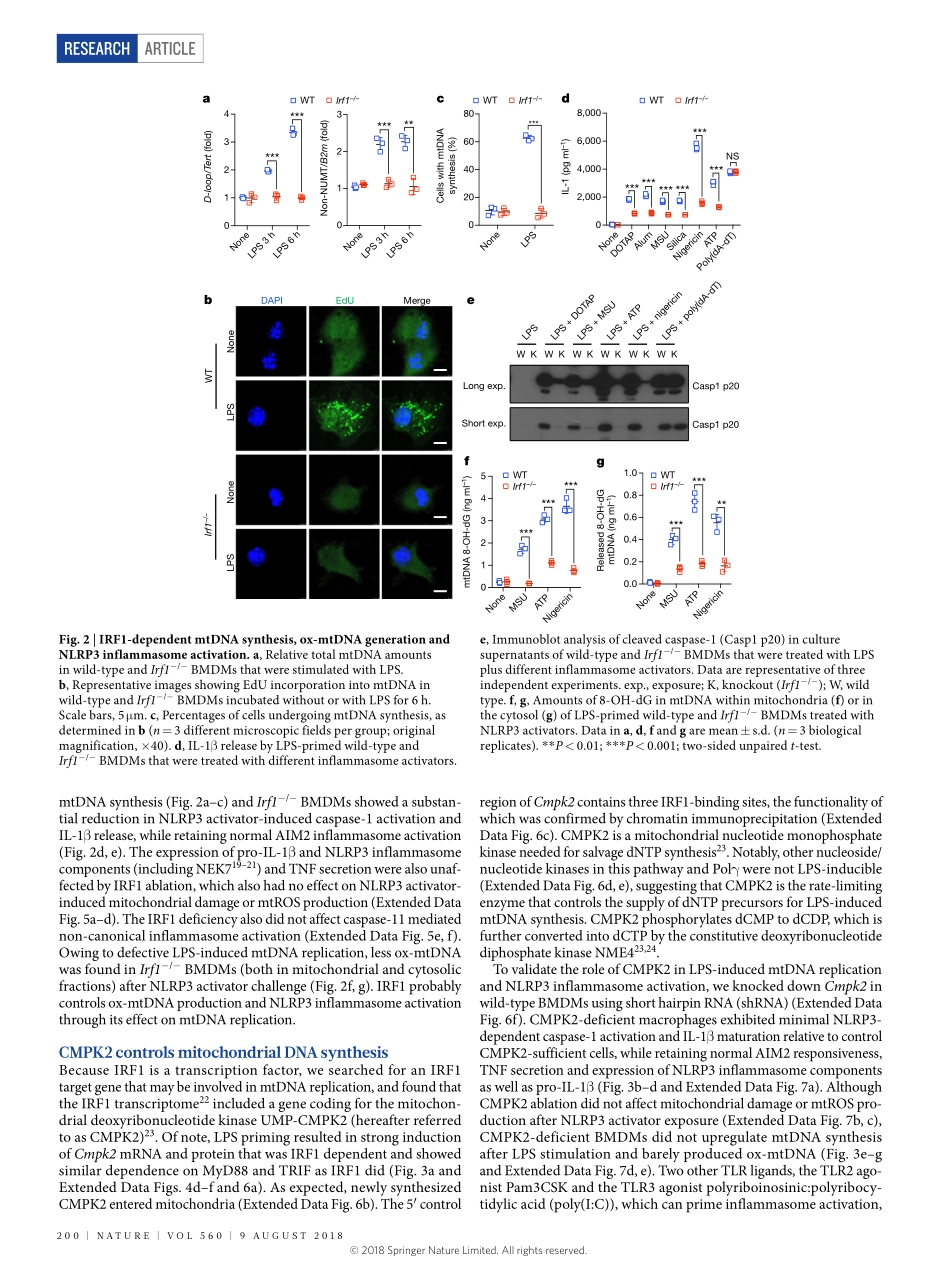
Articlehttps://doi.org/10.1038/s41586-018-0372-zNewmitochondrialDNAsynthesisenablesNLRP3inflammasomeactivationZhenyuZhong1,2,11,Shuangliang3,4,11,elsaSanchez-lopez1,2,FengHe1,2,ShabnamShalapour1,2,Xue-jialin1,2,5,JerryWong1,2,SiyuanDing6,7,8,ekihiroSeki9,BerndSchnabl3,Andreal.Hevener10,HarryB.Greenberg6,7,8,tatianaKisseleva4&MichaelKarin1,2*DysregulatedNLRP3inflammasomeactivityresultsinuncontrolledinflammation,whichunderliesmanychronicdiseases.AlthoughmitochondrialdamageisneededfortheassemblyandactivationoftheNLRP3inflammasome,itisunclearhowmacrophagesareabletorespondtostructurallydiverseinflammasome-activatingstimuli.HereweshowthatthesynthesisofmitochondrialDNA(mtDNA),inducedaftertheengagementofToll-likereceptors,iscrucialforNLRP3signalling.Toll-likereceptorssignalviatheMyD88andTRIFadaptorstotriggerIRF1-dependenttranscriptionofCMPK2,arate-limitingenzymethatsuppliesdeoxyribonucleotidesformtDNAsynthesis.CMPK2-dependentmtDNAsynthesisisnecessaryfortheproductionofoxidizedmtDNAfragmentsafterexposuretoNLRP3activators.CytosolicoxidizedmtDNAassociateswiththeNLRP3inflammasomecomplexandisrequiredforitsactivation.ThedependenceonCMPK2catalyticactivityprovidesopportunitiesformoreeffectivecontrolofNLRP3inflammasome-associateddiseases.Inflammationisinitiatedbythesensingofpathogen-associatedordamage-associatedmolecularpatterns1,2.Amongpattern-recognitionreceptors,NLRP3isuniqueinitsresponsetohighlydiverseextracel-lularstimuli,severalofwhichlinktissuedamagetosterileinflamma-tion,thegoalofwhichisdamagerepair3,4.Afterstimulation,NLRP3isthoughttoexposeitspyrindomain,whichbindsASC(apoptosis-associatedspeck-likeprotein)thatrecruitstheeffectormoleculepro-caspase-1viaCARD–CARDinteractionstoformalargecytosoliccomplex—theNLRP3inflammasome1,2,5.Inflammasomeassemblytriggerstheself-cleavageandactivationofcaspase-1,convertingpro-IL-1βandpro-IL-18totheirmatureforms1.PersistentandaberrantNLRP3signallingunderliesmanychronicanddegenerativediseases,includingperiodicauto-inflammatorysyndromes,gou...