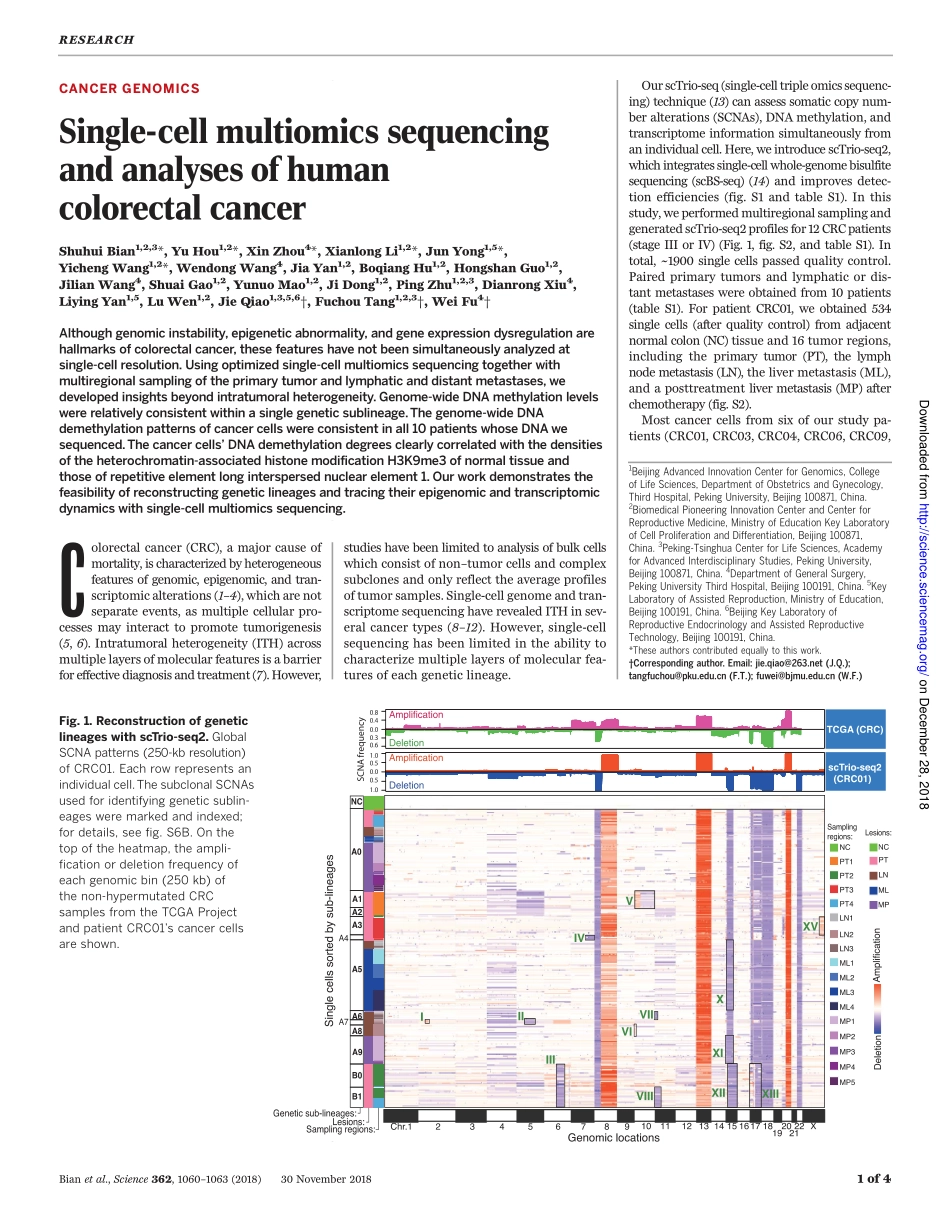
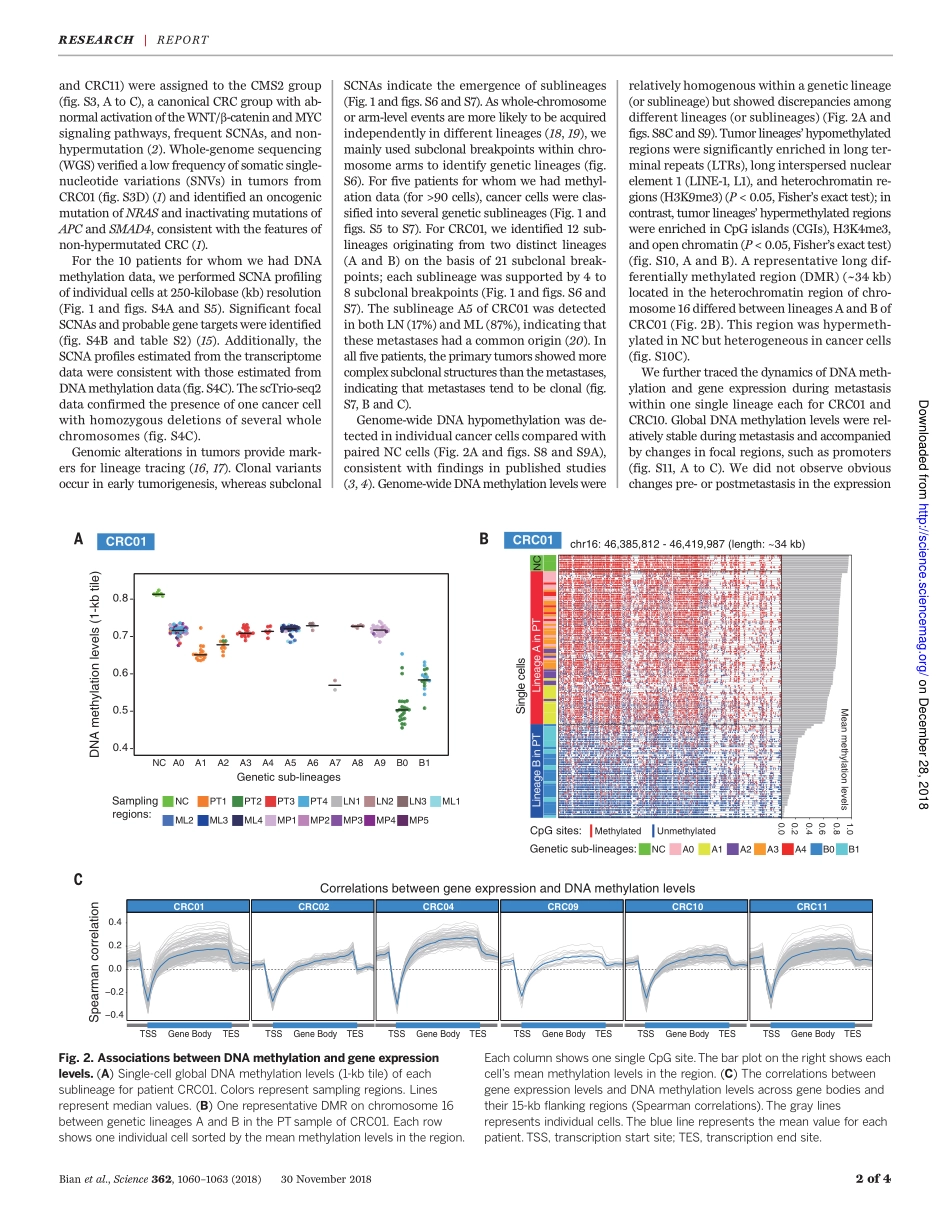
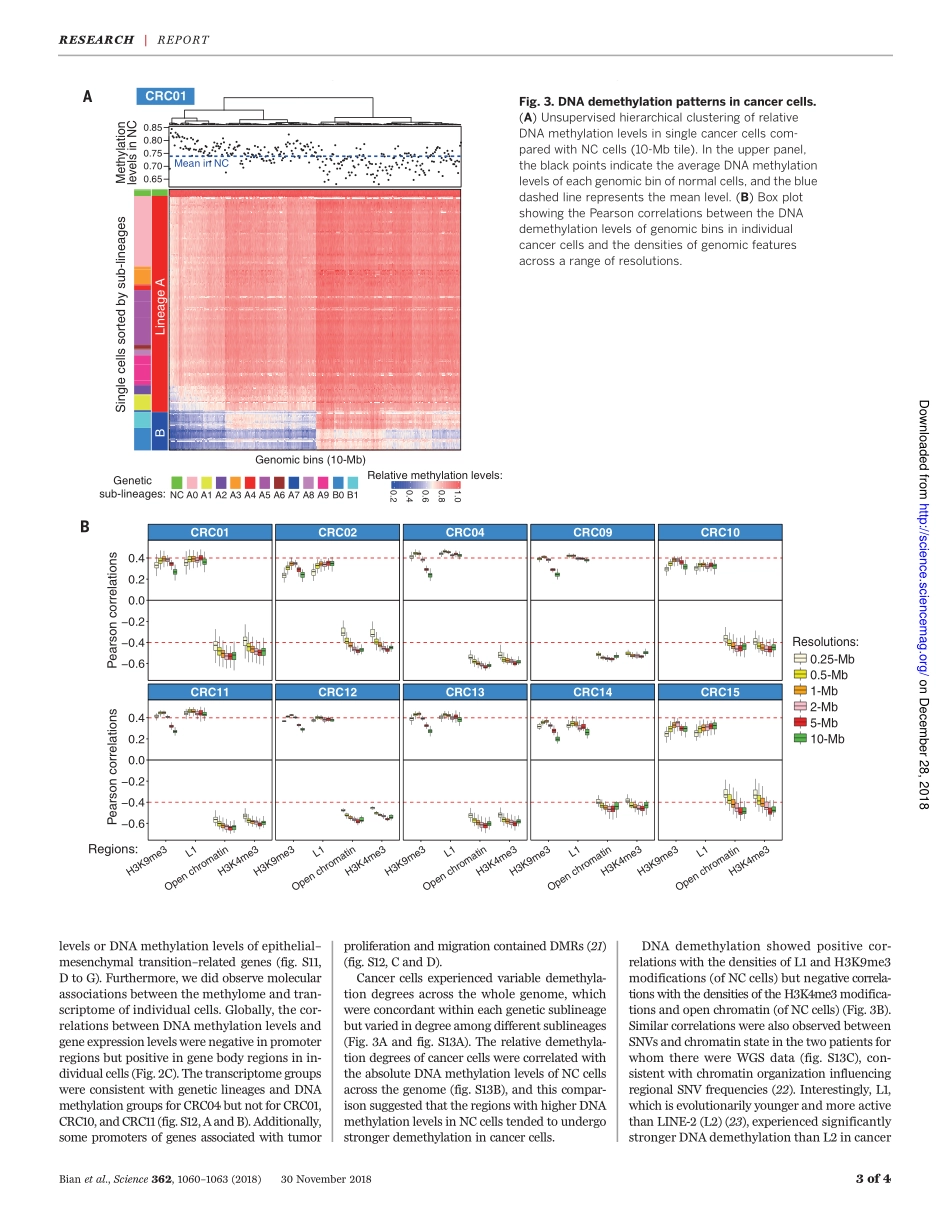
CANCERGENOMICSSingle-cellmultiomicssequencingandanalysesofhumancolorectalcancerShuhuiBian1,2,3*,YuHou1,2*,XinZhou4*,XianlongLi1,2*,JunYong1,5*,YichengWang1,2*,WendongWang4,JiaYan1,2,BoqiangHu1,2,HongshanGuo1,2,JilianWang4,ShuaiGao1,2,YunuoMao1,2,JiDong1,2,PingZhu1,2,3,DianrongXiu4,LiyingYan1,5,LuWen1,2,JieQiao1,3,5,6†,FuchouTang1,2,3†,WeiFu4†Althoughgenomicinstability,epigeneticabnormality,andgeneexpressiondysregulationarehallmarksofcolorectalcancer,thesefeatureshavenotbeensimultaneouslyanalyzedatsingle-cellresolution.Usingoptimizedsingle-cellmultiomicssequencingtogetherwithmultiregionalsamplingoftheprimarytumorandlymphaticanddistantmetastases,wedevelopedinsightsbeyondintratumoralheterogeneity.Genome-wideDNAmethylationlevelswererelativelyconsistentwithinasinglegeneticsublineage.Thegenome-wideDNAdemethylationpatternsofcancercellswereconsistentinall10patientswhoseDNAwesequenced.Thecancercells’DNAdemethylationdegreesclearlycorrelatedwiththedensitiesoftheheterochromatin-associatedhistonemodificationH3K9me3ofnormaltissueandthoseofrepetitiveelementlonginterspersednuclearelement1.Ourworkdemonstratesthefeasibilityofreconstructinggeneticlineagesandtracingtheirepigenomicandtranscriptomicdynamicswithsingle-cellmultiomicssequencing.Colorectalcancer(CRC),amajorcauseofmortality,ischaracterizedbyheterogeneousfeaturesofgenomic,epigenomic,andtran-scriptomicalterations(1–4),whicharenotseparateevents,asmultiplecellularpro-cessesmayinteracttopromotetumorigenesis(5,6).Intratumoralheterogeneity(ITH)acrossmultiplelayersofmolecularfeaturesisabarrierforeffectivediagnosisandtreatment(7).However,studieshavebeenlimitedtoanalysisofbulkcellswhichconsistofnon–tumorcellsandcomplexsubclonesandonlyreflecttheaverageprofilesoftumorsamples.Single-cellgenomeandtran-scriptomesequencinghaverevealedITHinsev-eralcancertypes(8–12).However,single-cellsequencinghasbeenlimitedintheabilitytocharacterizemultiplelayersofmolecularfea-turesofeachgeneticlineage.OurscTrio-seq(single-celltripleomicssequenc-in...