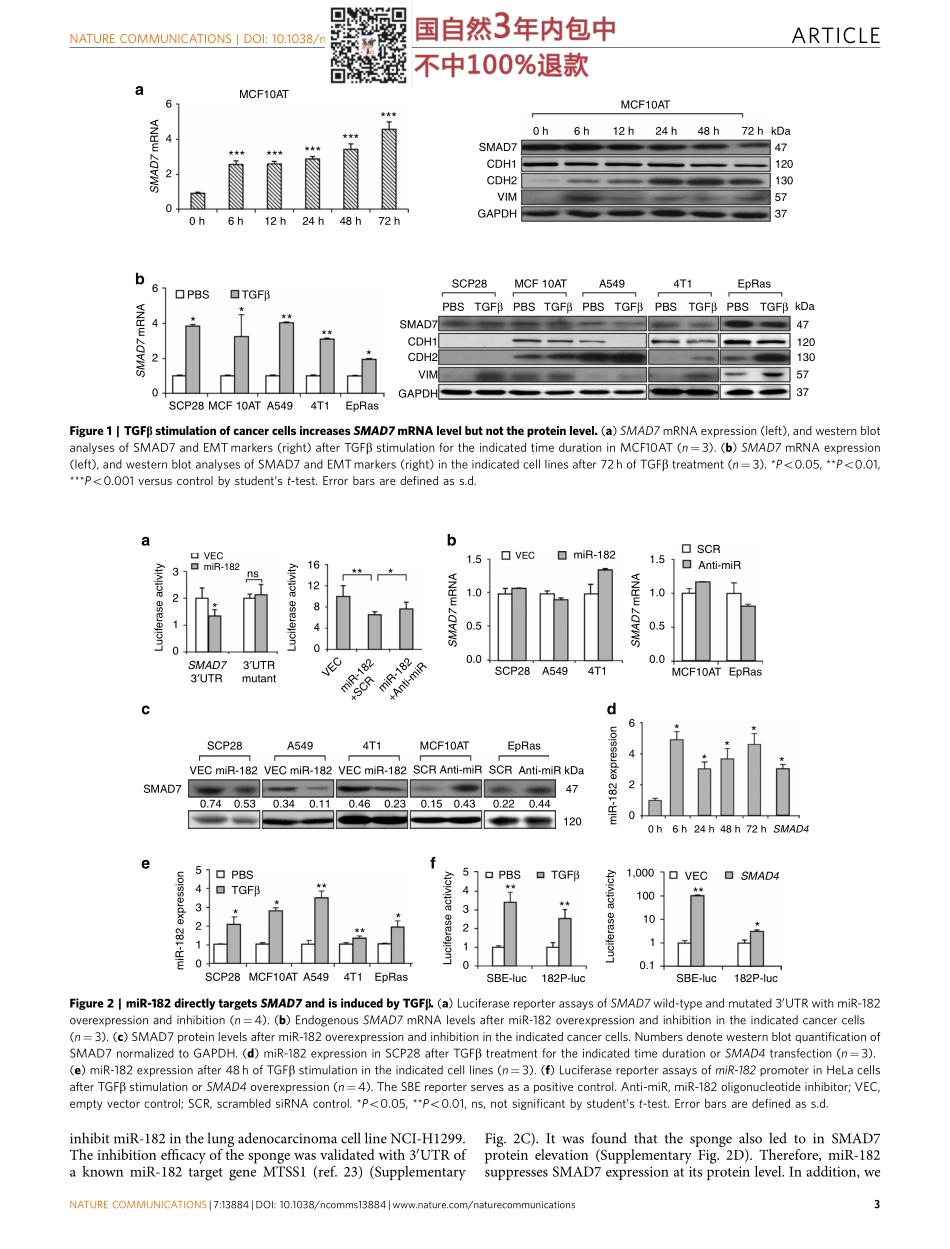
ARTICLEReceived2Nov2015|Accepted9Nov2016|Published20Dec2016MicroRNA-182targetsSMAD7topotentiateTGFb-inducedepithelial-mesenchymaltransitionandmetastasisofcancercellsJingyiYu1,*,RongLei1,*,XueqianZhuang1,XiaoxunLi1,GangLi1,SimaLev2,MiguelF.Segura3,w,XueZhang1&GuohongHu1Thetransforminggrowthfactorb(TGFb)pathwayplayscriticalrolesduringcancercellepithelial-mesenchymaltransition(EMT)andmetastasis.SMAD7isbothatranscriptionaltargetandanegativeregulatorofTGFbsignalling,thusmediatinganegativefeedbackloopthatmaypotentiallyrestrainTGFbresponsesofcancercells.Here,however,weshowthatTGFbtreatmentinducesSMAD7transcriptionbutnotitsproteinlevelinapanelofcancercells.MechanisticstudiesrevealthatTGFbactivatestheexpressionofmicroRNA-182(miR-182),whichsuppressesSMAD7protein.miR-182silencingleadstoSMAD7upregulationonTGFbtreatmentandpreventsTGFb-inducedEMTandinvasionofcancercells.OverexpressionofmiR-182promotesbreasttumourinvasionandTGFb-inducedosteoclastogenesisforbonemetastasis.Furthermore,miR-182expressioninverselycorrelateswithSMAD7proteininhumantumoursamples.Therefore,ourdatarevealthemiR-182-mediateddisruptionofTGFbself-restraintandprovideamechanismtoexplaintheunleashedTGFbresponsesinmetastaticcancercells.DOI:10.1038/ncomms13884OPEN1TheKeyLaboratoryofStemCellBiology,InstituteofHealthSciences,ShanghaiInstitutesforBiologicalSciences,ChineseAcademyofSciences&ShanghaiJiaoTongUniversitySchoolofMedicine,UniversityofChineseAcademyofSciences,Shanghai200031,China.2MolecularCellBiologyDepartment,WeizmannInstituteofScience,Rehovot76100,Israel.3DepartmentofPathology,NewYorkUniversitySchoolofMedicine,NewYork,NewYork10016,USA.*Theseauthorscontributedequallytothiswork.wPresentaddress:LaboratoryofTranslationalResearchinAdolescentandChildhoodCancer,Valld’HebronResearchInstitute(VHIR)-UAB,Barcelona119129-08035,Spain.CorrespondenceandrequestsformaterialsshouldbeaddressedtoG.H.(email:ghhu@sibs.ac.cn).NATURECOMMUNICATIONS|7:13884|DOI:10.1038/ncomms13884|www.nature.com/naturecommunications1TheTGFbpathw...