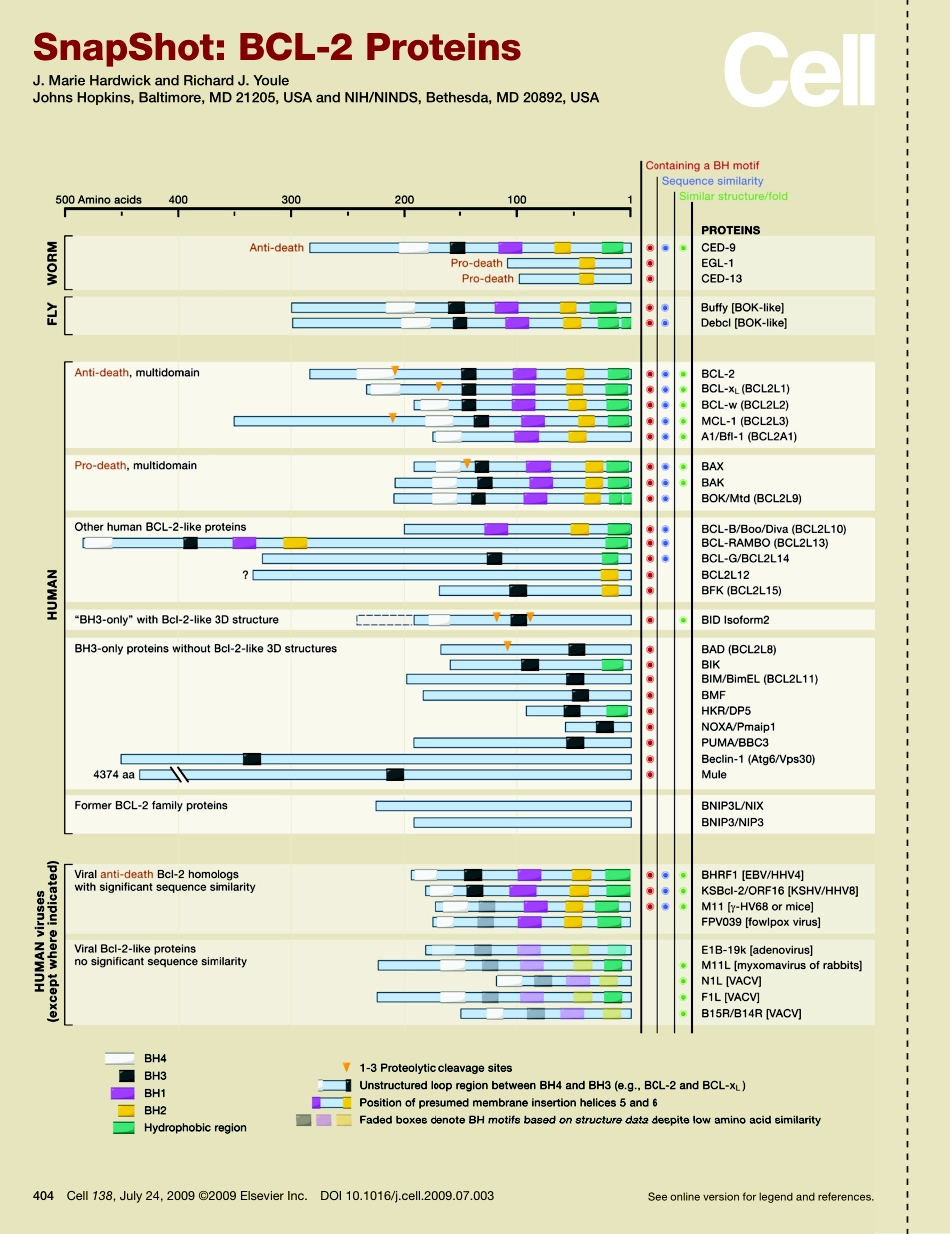
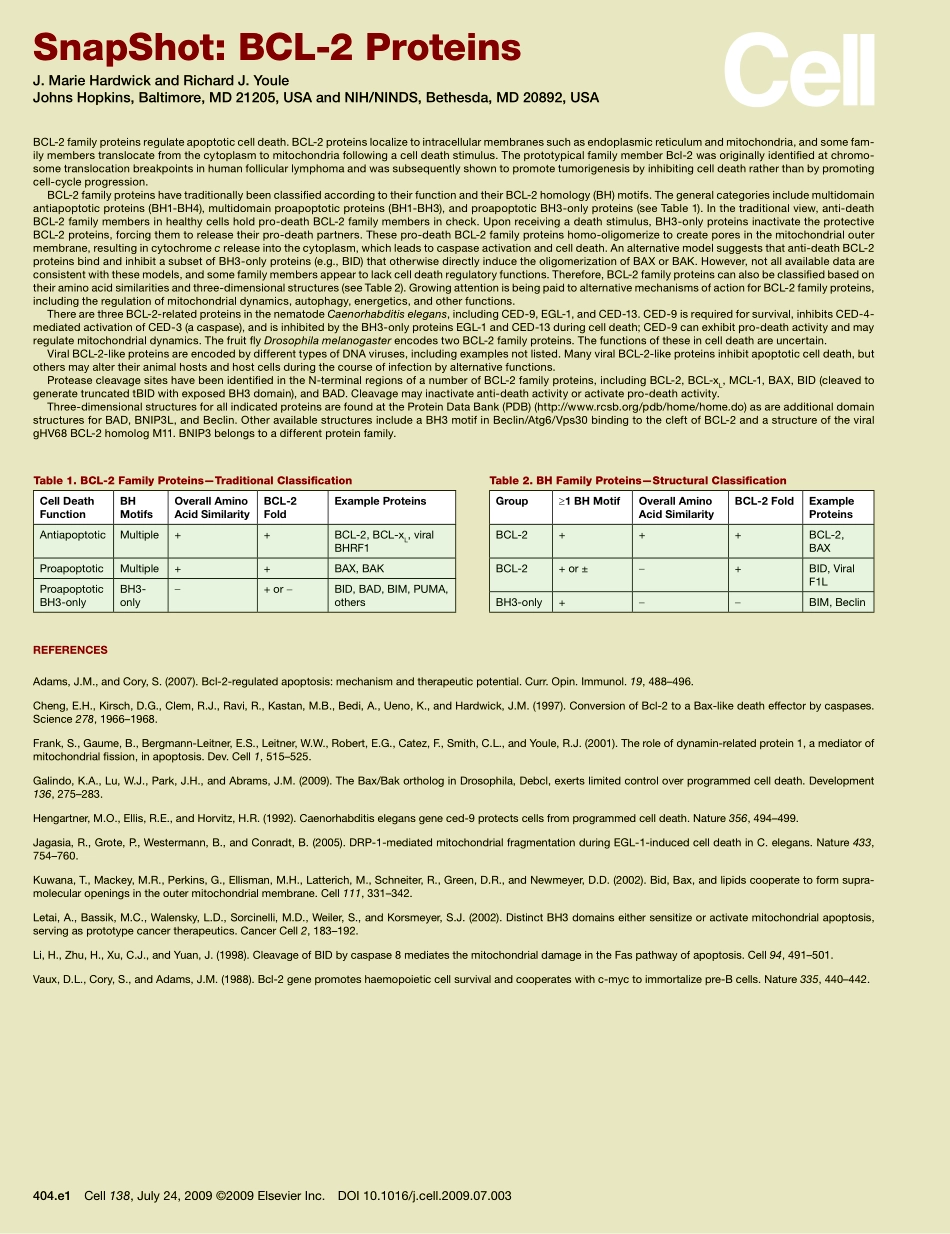
SnapShot:BCL-2ProteinsJ.MarieHardwickandRichardJ.YouleJohnsHopkins,Baltimore,MD21205,USAandNIH/NINDS,Bethesda,MD20892,USASeeonlineversionforlegendandreferences.404Cell138,July24,2009©2009ElsevierInc.DOI10.1016/j.cell.2009.07.003SnapShot:BCL-2ProteinsJ.MarieHardwickandRichardJ.YouleJohnsHopkins,Baltimore,MD21205,USAandNIH/NINDS,Bethesda,MD20892,USA404.e1Cell138,July24,2009©2009ElsevierInc.DOI10.1016/j.cell.2009.07.003BCL-2familyproteinsregulateapoptoticcelldeath.BCL-2proteinslocalizetointracellularmembranessuchasendoplasmicreticulumandmitochondria,andsomefam-ilymemberstranslocatefromthecytoplasmtomitochondriafollowingacelldeathstimulus.TheprototypicalfamilymemberBcl-2wasoriginallyidentifiedatchromo-sometranslocationbreakpointsinhumanfollicularlymphomaandwassubsequentlyshowntopromotetumorigenesisbyinhibitingcelldeathratherthanbypromotingcell-cycleprogression.BCL-2familyproteinshavetraditionallybeenclassifiedaccordingtotheirfunctionandtheirBCL-2homology(BH)motifs.Thegeneralcategoriesincludemultidomainantiapoptoticproteins(BH1-BH4),multidomainproapoptoticproteins(BH1-BH3),andproapoptoticBH3-onlyproteins(seeTable1).Inthetraditionalview,anti-deathBCL-2familymembersinhealthycellsholdpro-deathBCL-2familymembersincheck.Uponreceivingadeathstimulus,BH3-onlyproteinsinactivatetheprotectiveBCL-2proteins,forcingthemtoreleasetheirpro-deathpartners.Thesepro-deathBCL-2familyproteinshomo-oligomerizetocreateporesinthemitochondrialoutermembrane,resultingincytochromecreleaseintothecytoplasm,whichleadstocaspaseactivationandcelldeath.Analternativemodelsuggeststhatanti-deathBCL-2proteinsbindandinhibitasubsetofBH3-onlyproteins(e.g.,BID)thatotherwisedirectlyinducetheoligomerizationofBAXorBAK.However,notallavailabledataareconsistentwiththesemodels,andsomefamilymembersappeartolackcelldeathregulatoryfunctions.Therefore,BCL-2familyproteinscanalsobeclassifiedbasedontheiraminoacidsimilaritiesandthree-dimensionalstructures(seeTable2).Growingattentionisbeingpaidtoalternativemechanismsofactionfor...