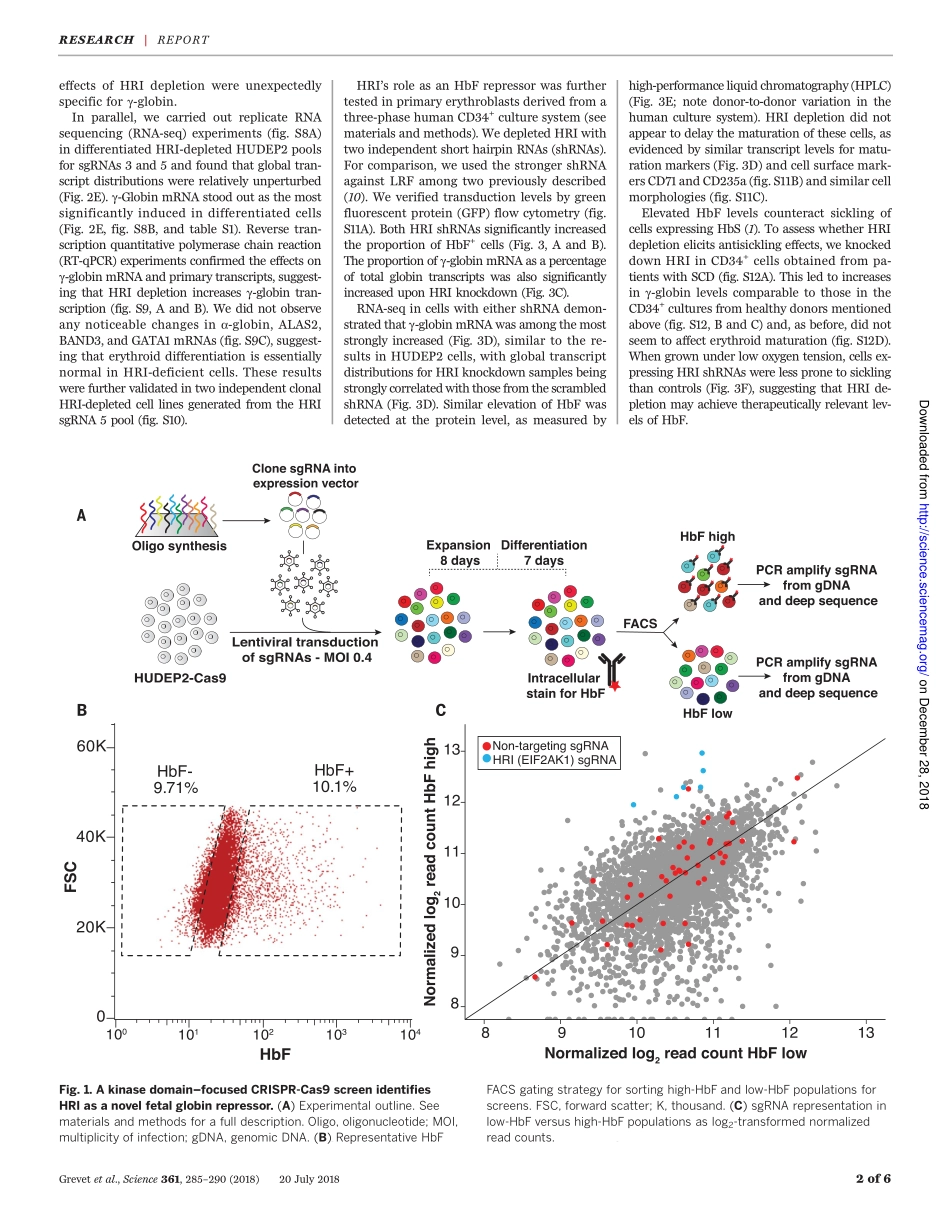
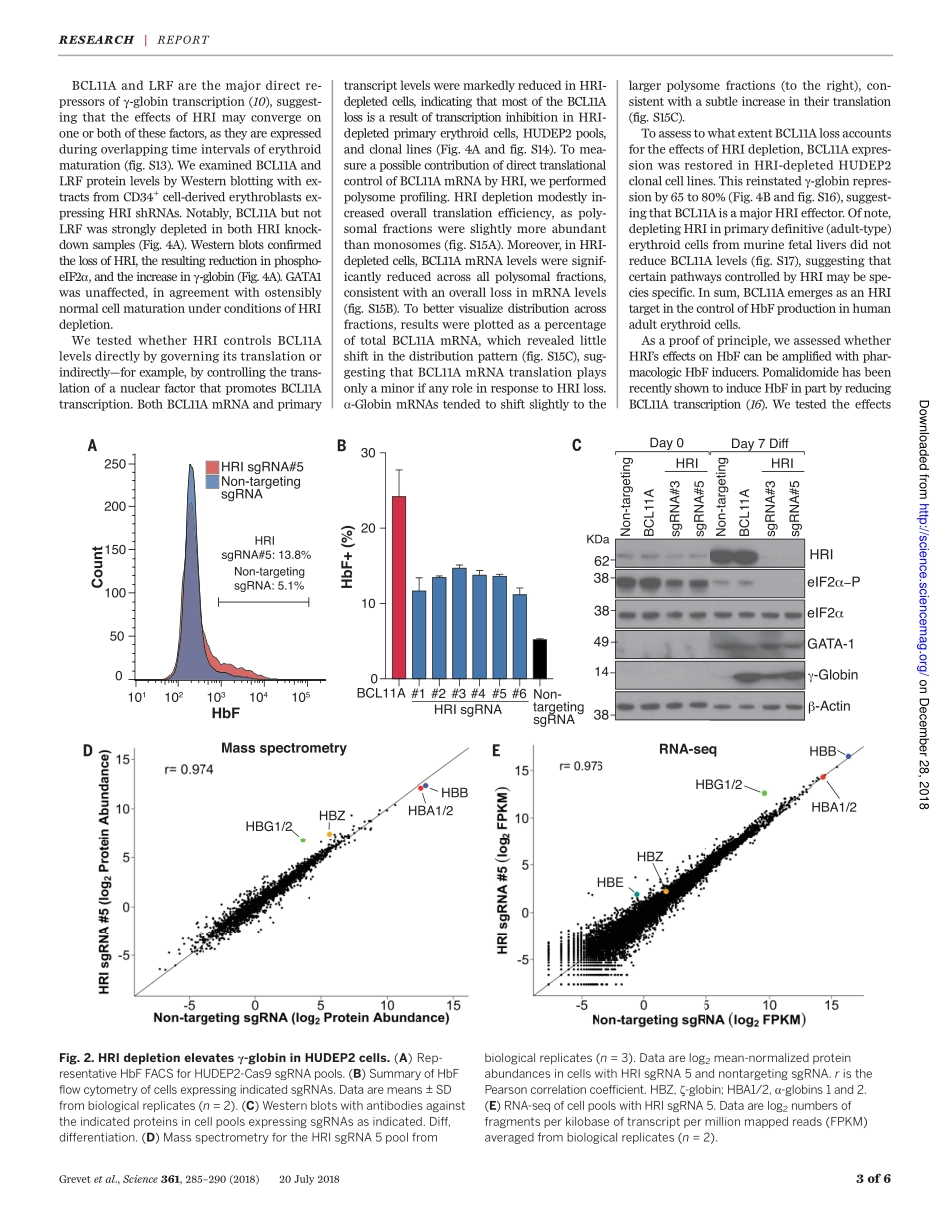
REDBLOODCELLSDomain-focusedCRISPRscreenidentifiesHRIasafetalhemoglobinregulatorinhumanerythroidcellsJeremyD.Grevet1,2*,XianjiangLan1*,NicoleHamagami1,ChristopherR.Edwards1,LaavanyaSankaranarayanan1,XinjunJi2,SaurabhK.Bhardwaj1,CarolyneJ.Face1,DavidF.Posocco1,OsheizaAbdulmalik1,CherylA.Keller3,BelindaGiardine3,SimoneSidoli4,BenA.Garcia4,StellaT.Chou1,StephenA.Liebhaber2,RossC.Hardison3,JunweiShi5†,GerdA.Blobel1,2†Increasingfetalhemoglobin(HbF)levelsinadultredbloodcellsprovidesclinicalbenefittopatientswithsicklecelldiseaseandsomeformsofb-thalassemia.ToidentifypotentiallydruggableHbFregulatorsinadulthumanerythroidcells,weemployedaproteinkinasedomain–focusedCRISPR-Cas9–basedgeneticscreenwithanewlyoptimizedsingle-guideRNAscaffold.Thescreenuncoveredtheheme-regulatedinhibitorHRI(alsoknownasEIF2AK1),anerythroid-specifickinasethatcontrolsproteintranslation,asanHbFrepressor.HRIdepletionmarkedlyincreasedHbFproductioninaspecificmannerandreducedsicklinginculturederythroidcells.DiminishedexpressionoftheHbFrepressorBCL11AaccountedinlargepartfortheeffectsofHRIdepletion.Takentogether,theseresultssuggestHRIasapotentialtherapeutictargetforhemoglobinopathies.Thehumanb-globingeneclustercomprisesanembryonic[e-globin(HBE)],twofetal[g-globin(HBG1andHBG2)],andtwoadult-type[d-globinandb-globin(HBDandHBB)]genes(1).Diseasesaffectingtheadult-typeb-globingenes,suchassicklecelldisease(SCD)andsometypesofb-thalassemia,manifestthem-selvesafterbirthfollowingthetransitionfromfetaltoadulthemoglobinproduction.Theclin-icalcourseofthesediseasesisamelioratedwhenfetalhemoglobin(HbF)levelsareelevatedbe-causeofgeneticcausesormedicalintervention(2).Genome-wideassociationstudieshaveiden-tifiedthreemajorlocithatarelinkedtonat-uralvariationinHbFlevels,includingthatofthetranscriptionfactorBCL11A(1).PatientswithheterozygouslossofBCL11AexhibitelevatedHbFlevelsthroughoutadultlife(3),andBCL11Adepletioninculturedhumanerythroidprecur-sorsraisesHbFproduction(4,5).LossofBCL11Ainengineeredmicethatexpresshumansickleh...