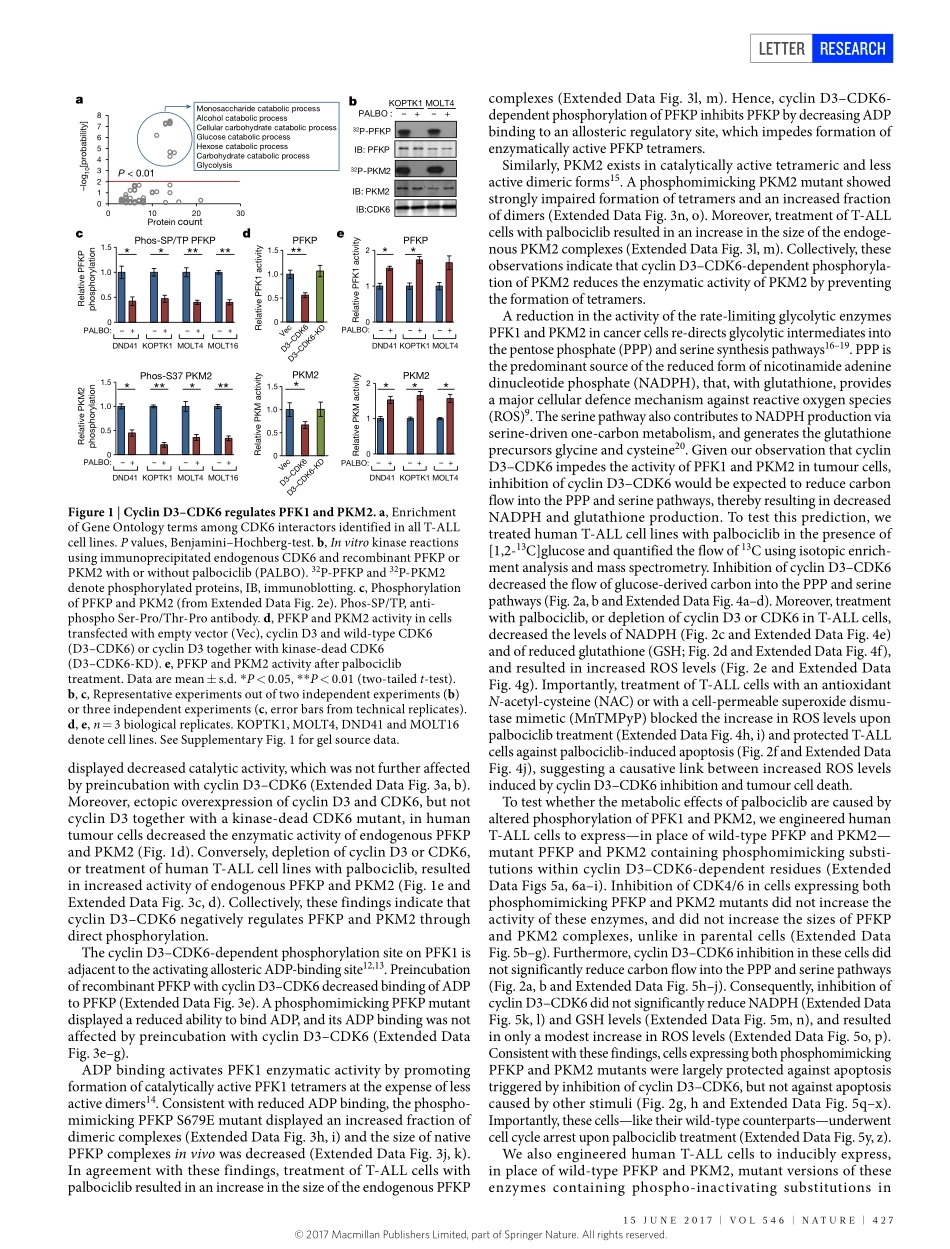
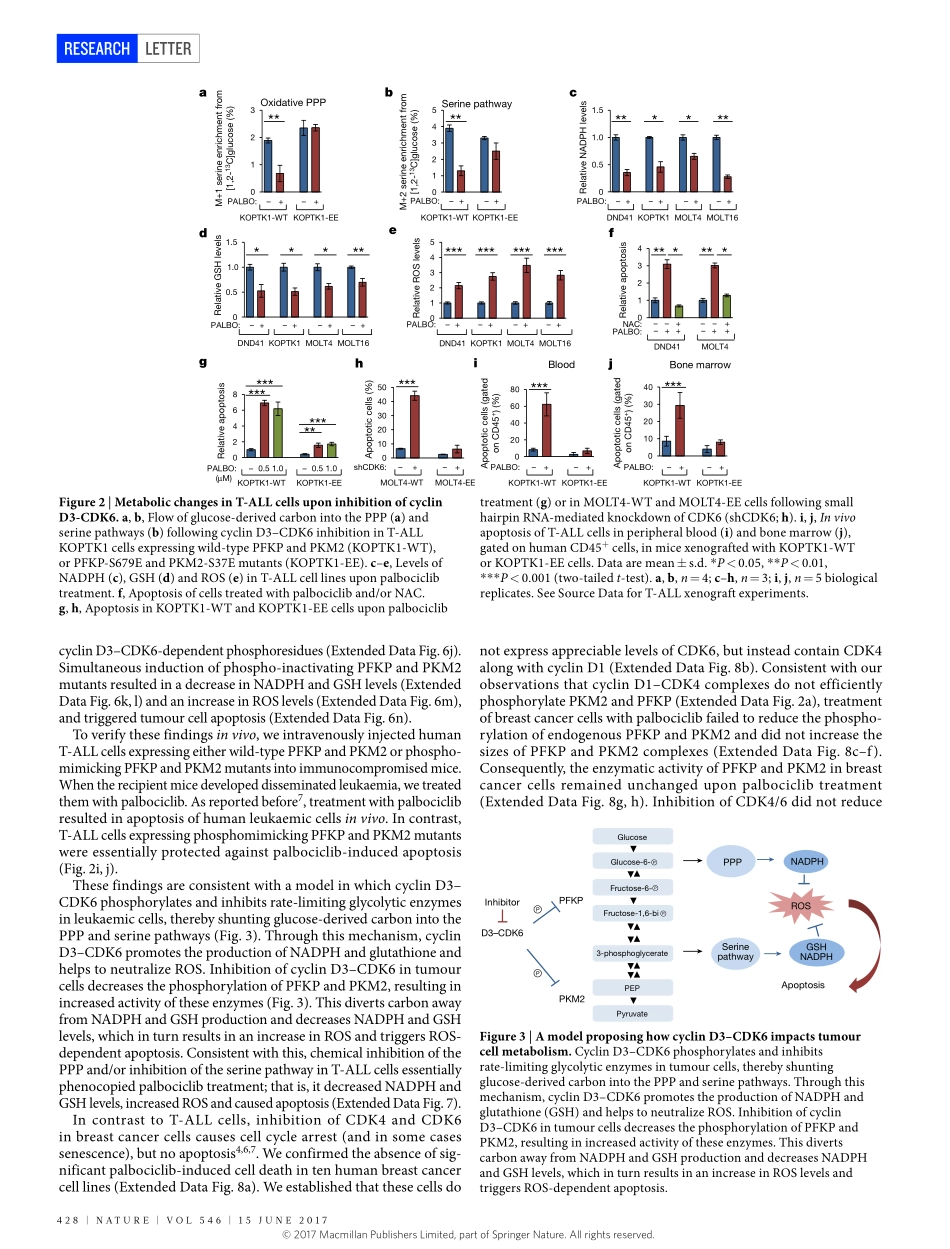
426|NATURE|VOL546|15JUNE2017LETTERdoi:10.1038/nature22797ThemetabolicfunctionofcyclinD3–CDK6kinaseincancercellsurvivalHaizhenWang1,2,BrandonN.Nicolay3,JoelM.Chick4,XueliangGao1,5,YanGeng1,2,HongRen1,2,HuiGao6,GuizhiYang6,JulietA.Williams6,JanM.Suski1,2,MarkA.Keibler7,EwaSicinska8,UlrikeGerdemann9,W.NicholasHaining9,10,11,ThomasM.Roberts1,5,KorneliaPolyak12,StevenP.Gygi4,NicholasJ.Dyson3&PiotrSicinski1,2D-typecyclins(D1,D2andD3)andtheirassociatedcyclin-dependentkinases(CDK4andCDK6)arecomponentsofthecorecellcyclemachinerythatdrivescellproliferation1,2.InhibitorsofCDK4andCDK6arecurrentlybeingtestedinclinicaltrialsforpatientswithseveralcancertypes,withpromisingresults2.Here,usinghumancancercellsandpatient-derivedxenograftsinmice,weshowthatthecyclinD3–CDK6kinasephosphorylatesandinhibitsthecatalyticactivityoftwokeyenzymesintheglycolyticpathway,6-phosphofructokinaseandpyruvatekinaseM2.Thisre-directstheglycolyticintermediatesintothepentosephosphate(PPP)andserinepathways.InhibitionofcyclinD3–CDK6intumourcellsreducesflowthroughthePPPandserinepathways,therebydepletingtheantioxidantsNADPHandglutathione.This,inturn,increasesthelevelsofreactiveoxygenspeciesandcausesapoptosisoftumourcells.Thepro-survivalfunctionofcyclinD-associatedkinaseoperatesintumoursexpressinghighlevelsofcyclinD3–CDK6complexes.WeproposethatmeasuringthelevelsofcyclinD3–CDK6inhumancancersmighthelptoidentifytumoursubsetsthatundergocelldeathandtumourregressionuponinhibitionofCDK4andCDK6.CyclinD3–CDK6,throughitsabilitytolinkcellcycleandcellmetabolism,representsaparticularlypowerfuloncoproteinthataffectscancercellsatseverallevels,andthispropertycanbeexploitedforanti-cancertherapy.D-typecyclins(D1,D2andD3)arecomponentsofthecorecellcyclemachinerythatactivatethecyclin-dependentkinasesCDK4andCDK6,andareoftenoverexpressedinhumancancers1–3.InhibitionofcyclinD–CDK4/6kinaseinretinoblastomaprotein(RB1)-proficientcancercellscausescellcyclearrestand,insomecases,cellularsenescence4,5.Bycontrast,tumourcellsthathavelos...