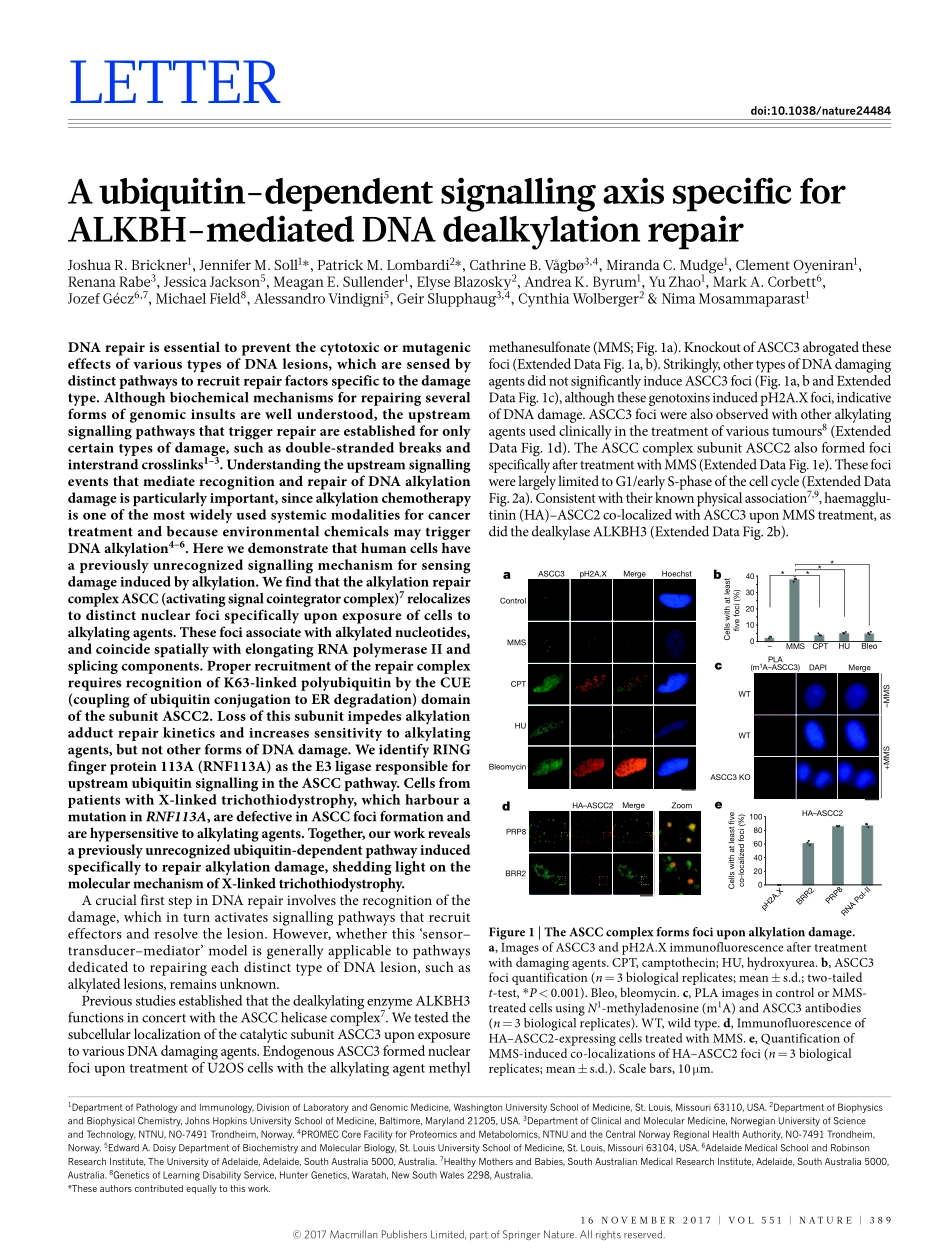
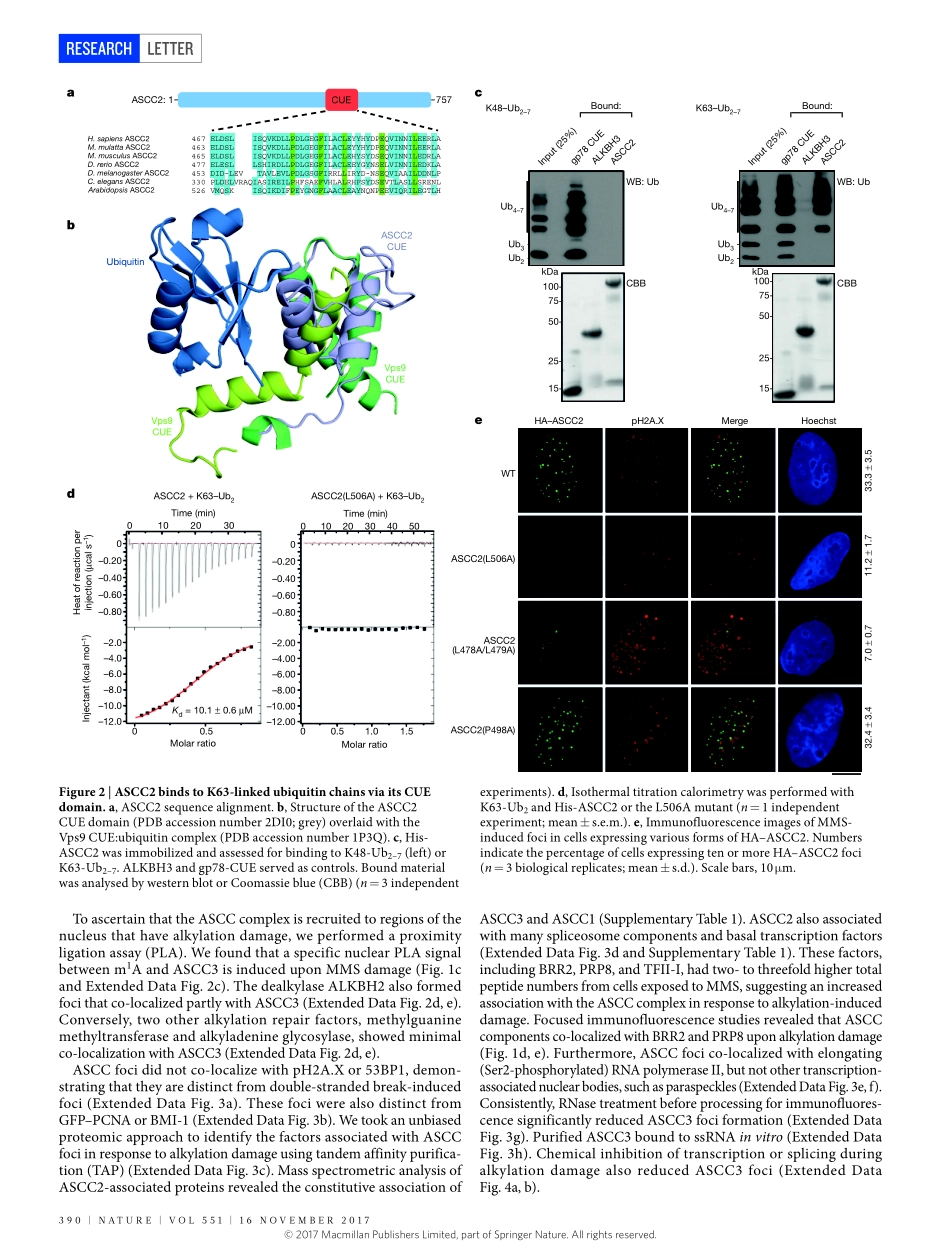
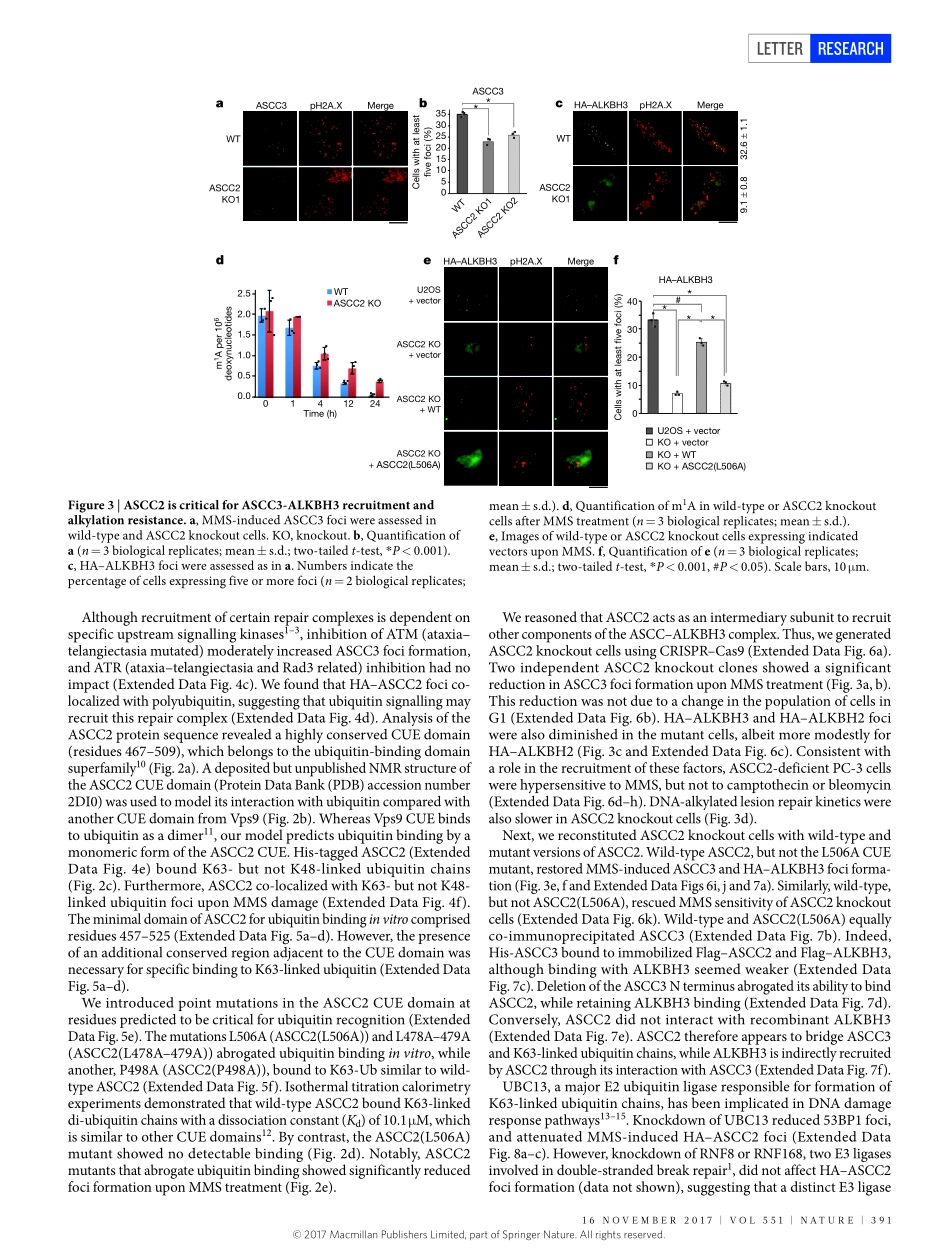
16november2017|voL551|nATUre|389LeTTerdoi:10.1038/nature24484Aubiquitin-dependentsignallingaxisspecificforALKBH-mediatedDNAdealkylationrepairJoshuar.brickner1,Jenniferm.Soll1*,Patrickm.Lombardi2*,Cathrineb.vågbø3,4,mirandaC.mudge1,Clementoyeniran1,renanarabe3,JessicaJackson5,meagane.Sullender1,elyseblazosky2,AndreaK.byrum1,YuZhao1,markA.Corbett6,JozefGécz6,7,michaelField8,Alessandrovindigni5,GeirSlupphaug3,4,CynthiaWolberger2&nimamosammaparast1DNArepairisessentialtopreventthecytotoxicormutageniceffectsofvarioustypesofDNAlesions,whicharesensedbydistinctpathwaystorecruitrepairfactorsspecifictothedamagetype.Althoughbiochemicalmechanismsforrepairingseveralformsofgenomicinsultsarewellunderstood,theupstreamsignallingpathwaysthattriggerrepairareestablishedforonlycertaintypesofdamage,suchasdouble-strandedbreaksandinterstrandcrosslinks1–3.UnderstandingtheupstreamsignallingeventsthatmediaterecognitionandrepairofDNAalkylationdamageisparticularlyimportant,sincealkylationchemotherapyisoneofthemostwidelyusedsystemicmodalitiesforcancertreatmentandbecauseenvironmentalchemicalsmaytriggerDNAalkylation4–6.Herewedemonstratethathumancellshaveapreviouslyunrecognizedsignallingmechanismforsensingdamageinducedbyalkylation.WefindthatthealkylationrepaircomplexASCC(activatingsignalcointegratorcomplex)7relocalizestodistinctnuclearfocispecificallyuponexposureofcellstoalkylatingagents.Thesefociassociatewithalkylatednucleotides,andcoincidespatiallywithelongatingRNApolymeraseIIandsplicingcomponents.ProperrecruitmentoftherepaircomplexrequiresrecognitionofK63-linkedpolyubiquitinbytheCUE(couplingofubiquitinconjugationtoERdegradation)domainofthesubunitASCC2.Lossofthissubunitimpedesalkylationadductrepairkineticsandincreasessensitivitytoalkylatingagents,butnototherformsofDNAdamage.WeidentifyRINGfingerprotein113A(RNF113A)astheE3ligaseresponsibleforupstreamubiquitinsignallingintheASCCpathway.CellsfrompatientswithX-linkedtrichothiodystrophy,whichharbouramutationinRNF113A,aredefectiveinASCCfociformat...