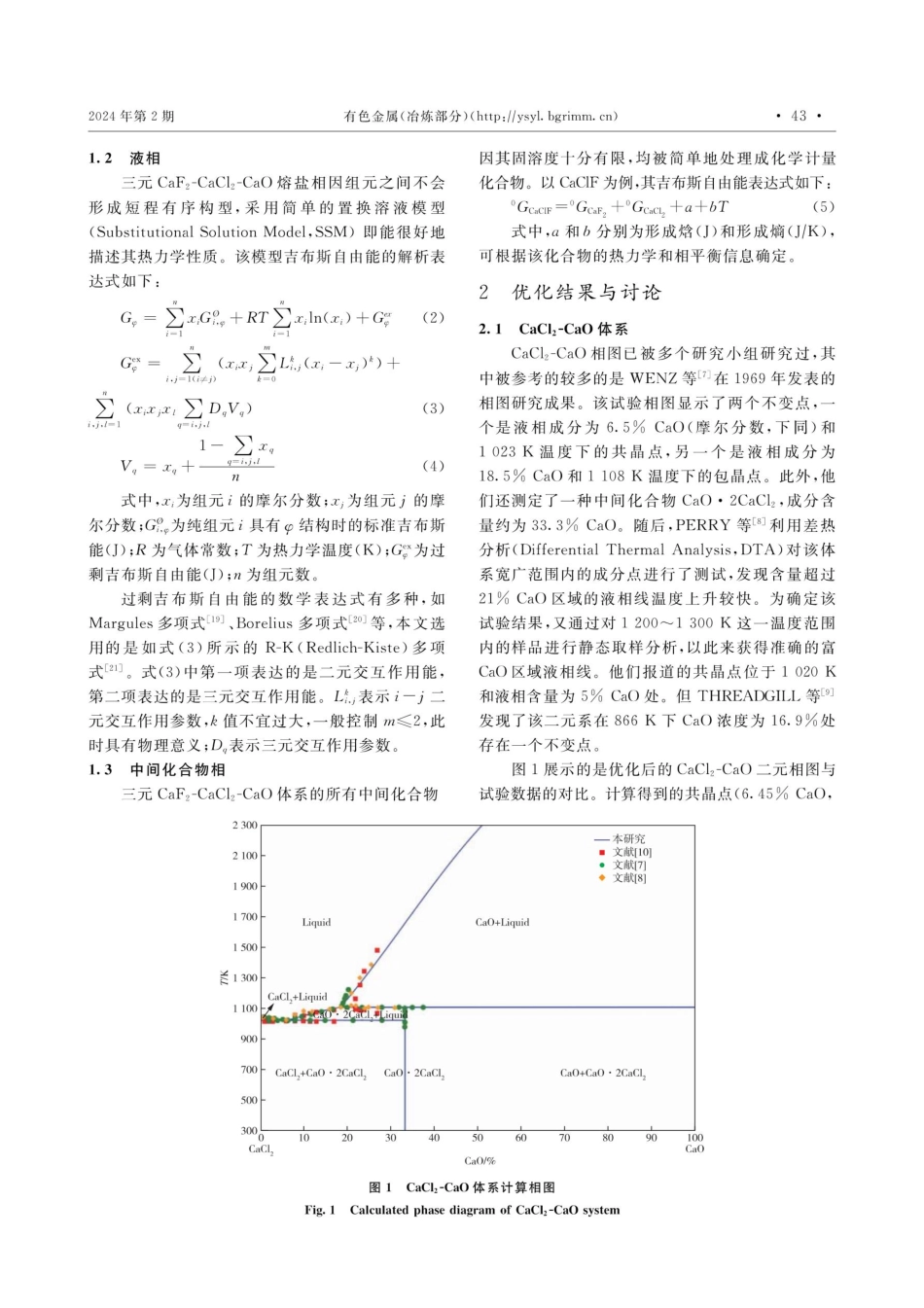
2024年第2期doi:10.3969/j.issn.1007-7545.2024.02.007有色金属(治炼部分)(http://ysyl.bgrimm.cn)CaCl²-CaF2-CaO三元体系的相图计算何艺霖,缪涵,庞忠亚,李光石,王坤,邹星礼,鲁雄刚(上海大学材料科学与工程学院,省部共建高品质特殊钢冶金与制备国家重点实验室,上海200444)摘要:基于Calphad方法,首先对CaCl2-CaO、CaCl2-CaF,和CaO-CaF2体系进行了系统的热力学评估和优化。采用置换溶液模型来描述液相和固溶体相的吉布斯自由能,所有中间相因其固溶区域十分有限而近似处理成准化学计量比化合物,且其自由能依据Neumann-Kopp规则定义。其次,利用Muggianu溶液几何模型将优化获得的所有子二元系的模型参数扩展至CaCl2-CaO-CaF2三元体系。最后,对共晶点进行配样和差示扫描量热法(DSC)测试,通过引入三元交互参数使计算结果与试验数据一致,从而获得了一套自洽的CaCl2-CaO-CaF,三元体系热力学数据库。关键词:相图;置换溶液模型;CaCl2-CaO-CaF2三元系;DSC中图分类号:TF827+.1文献标志码:A文章编号:1007-7545(2024)02-0041-07PhaseDiagramCalculationofCaCl,-CaF2-CaOSystemHEYilin,MIAOHan,PANGZhongya,LIGuangshi,WANGKun,ZOUXingli,LUXionggang(SchoolofMaterialScienceandEngineering,StateKeyLaboratoryofAdvancedSpecialSteel,ShanghaiUniversity,Shanghai200444,China)Abstract:Firstly,asystematicthermodynamicevaluationandoptimizationofCaCl2-CaO,CaCl2-CaF2andCaO-CaF2systemswerecarriedoutbasedontheCalphadmethod.ThesubstitutionsolutionmodelwasusedtodescribetheGibbsfreeenergyofliquidandsolidsolutionphases.Allintermediatephases,becauseoftheirlimitedsolidsolubility,wereapproximatelytreatedasstoichiometriccompoundsandtheirfreeenergiesweredefinedaccordingtotheNeumann-Kopprule.Secondly,theoptimizedmodelparametersofallsub-binarysystemswereextendedtotheCaCl,-CaF,-CaOternarysystembyusingtheMuggianugeometricsolutionmodel.Finally,sampleswiththeeutecticcompositionwerepreparedandthentestedbytheDifferentialScanningCalorimetry(DSC)method.Aself-consistentthermodynamicdatabaseofCaCl2-CaO-CaF2ternarysystemwasobtainedbyintroducingternaryinteractionparameterstomakethecalculationresultsconsistentwiththeexperimentaldata.Keywords:phasediagram;substitutionsoluti...