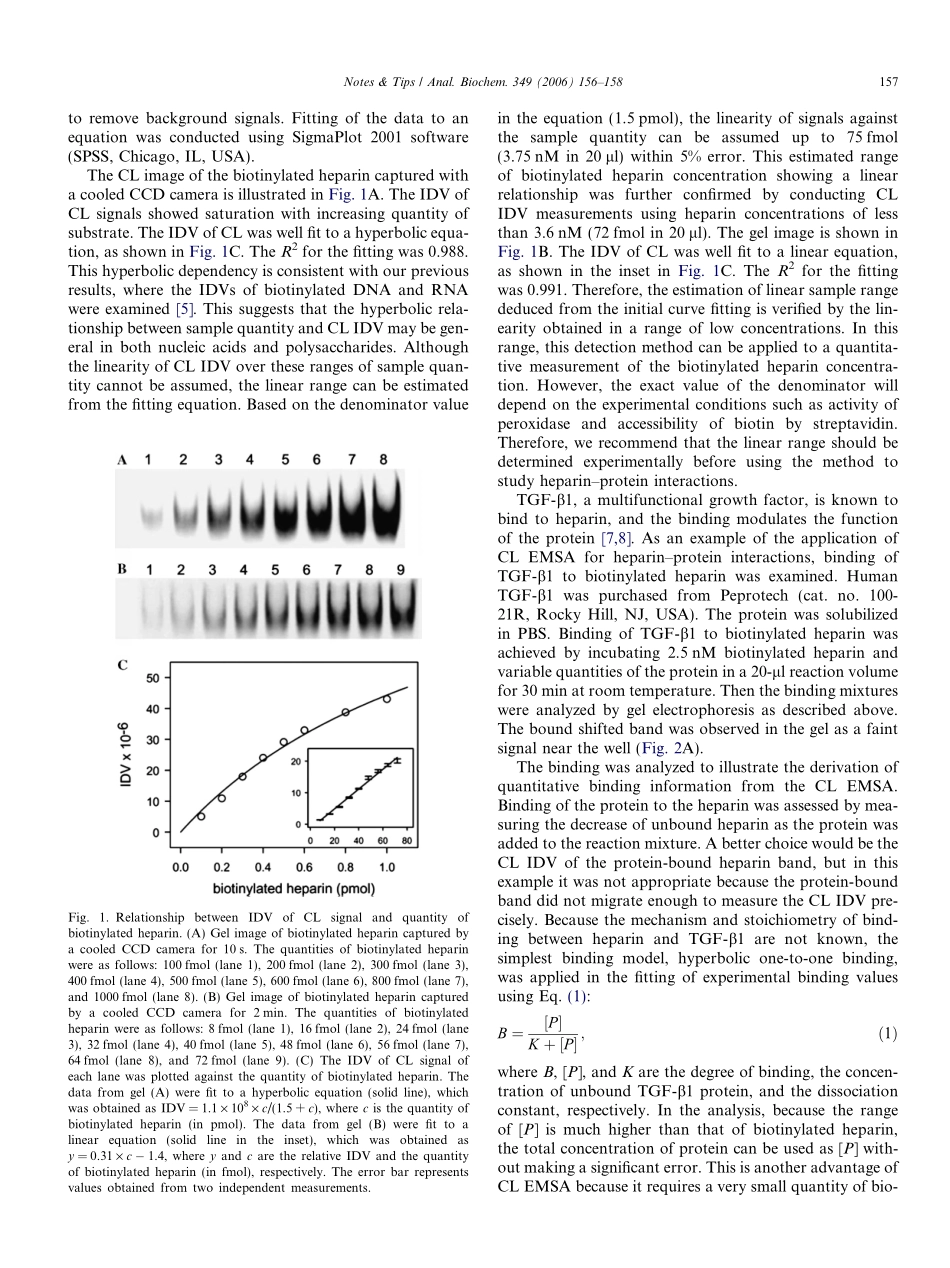
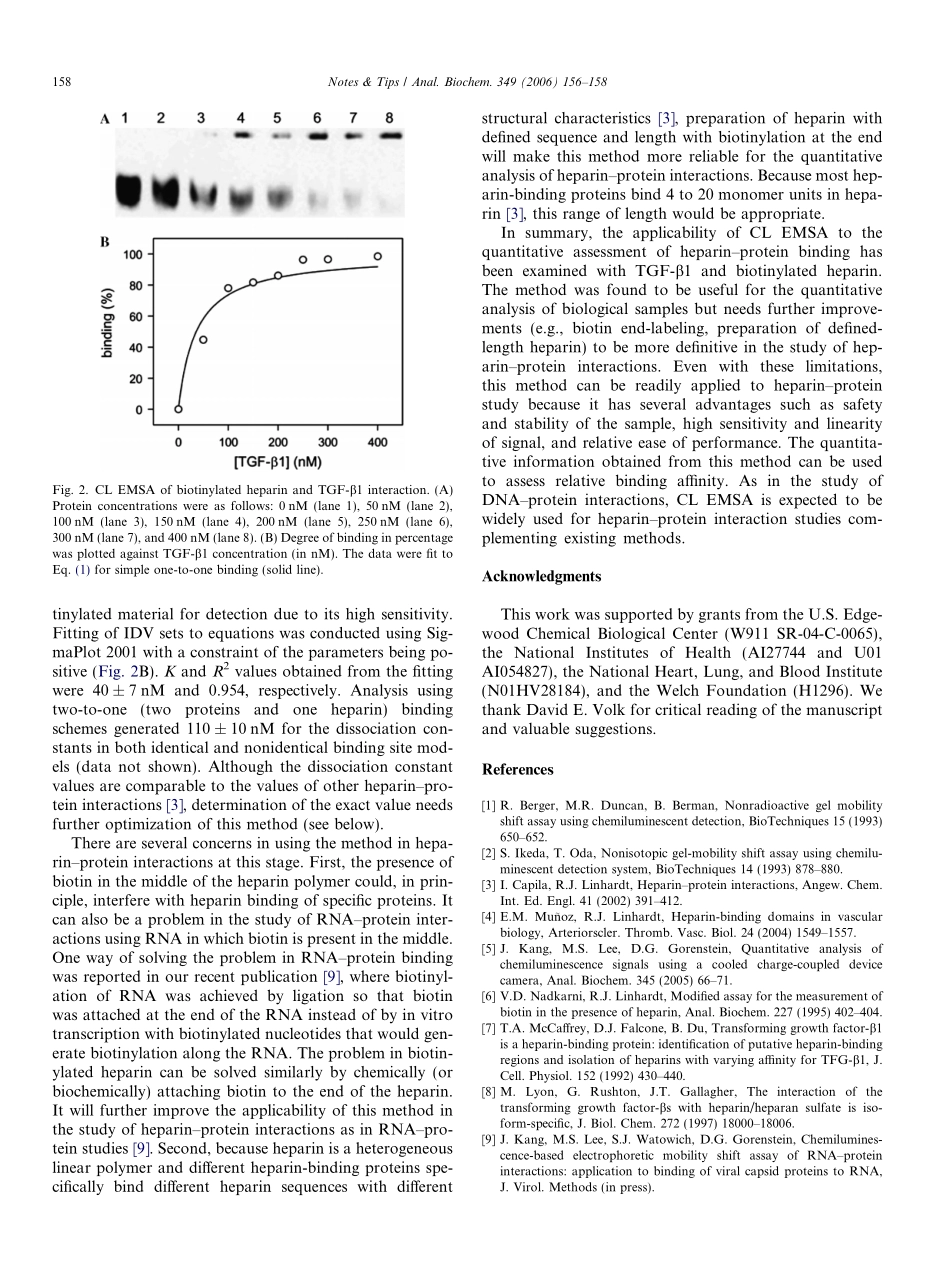
Notes&TipsChemiluminescence-basedelectrophoreticmobilityshiftassayofheparin–proteininteractionsJonghoonKang,MyungSoogLee,DavidG.Gorenstein*SealyCenterforStructuralBiologyandDepartmentofBiochemistryandMolecularBiology,UniversityofTexasMedicalBranch,Galveston,TX77555,USAReceived2August2005Availableonline30November2005Theelectrophoreticmobilityshiftassay(EMSA)1isoneofthemostsensitivemethodsforstudyingDNA–proteininteractions.Chemiluminescence(CL)hasbeenconsideredasanalternativetoradioisotopicdetectionofmaterialsintheEMSA[1,2]becauseithasadvantagessuchassafetyandstability(noisotopicdecay)ofthesample.Inthisstudy,weexaminedthefeasibilityoftheapplicationofCLEMSAtostudyingquantitativeheparin–proteininter-actions.Asanexampleofheparin-bindingproteins,trans-forminggrowthfactor(TGF)-b1wasexamined.Heparinisalinearpolysaccharideconsistingofrepeat-ingunitsofpyranosyluronicacidandglucosamineresidues[3].Asamemberofthefamilyofglycosaminoglycan,hep-arinperformsnumerousbiologicalfunctionsbyinteractingwithdiversevascularandplasmaproteins,includingprote-ases,growthfactors,chemokines,lipid-bindingproteins,pathogenproteins,andadhesionproteins[3,4].Withthediscoveryofincreasingnumbersofheparin-bindingpro-teins,severalmethodshavebeendevelopedtocharacterizethebindingofheparinwithproteins.Examplesofthesemethodsincludeaffinitychromatography,surfaceplasmonresonance,andisothermaltitrationcalorimetry[4].Becauseheparinhasahighnegativechargedensitywithanaverageof2.7negativechargesperdisacchariderepeatingunit[3],itcanbestudiedbypolyacrylamidegelelectrophoresis(PAGE).Therefore,itisfeasibletostudyheparin–proteininteractionsbyCLEMSA.Theunderly-inguseofCLEMSAforquantitativemeasurementscanbefoundinourrecentreport[5].Inbrief,biotinattachedtoheparinwasrecognizedbyhorseradishperoxidase-con-jugatedstreptavidin.Thisenzymecatalyzesachemicalreactiontogenerateluminescence.First,therelationshipbetweenCLintegrateddensityvalue(IDV)andquantityofbiotinylatedheparinwasexaminedtoevaluatethevalidityofthemethodinquan...