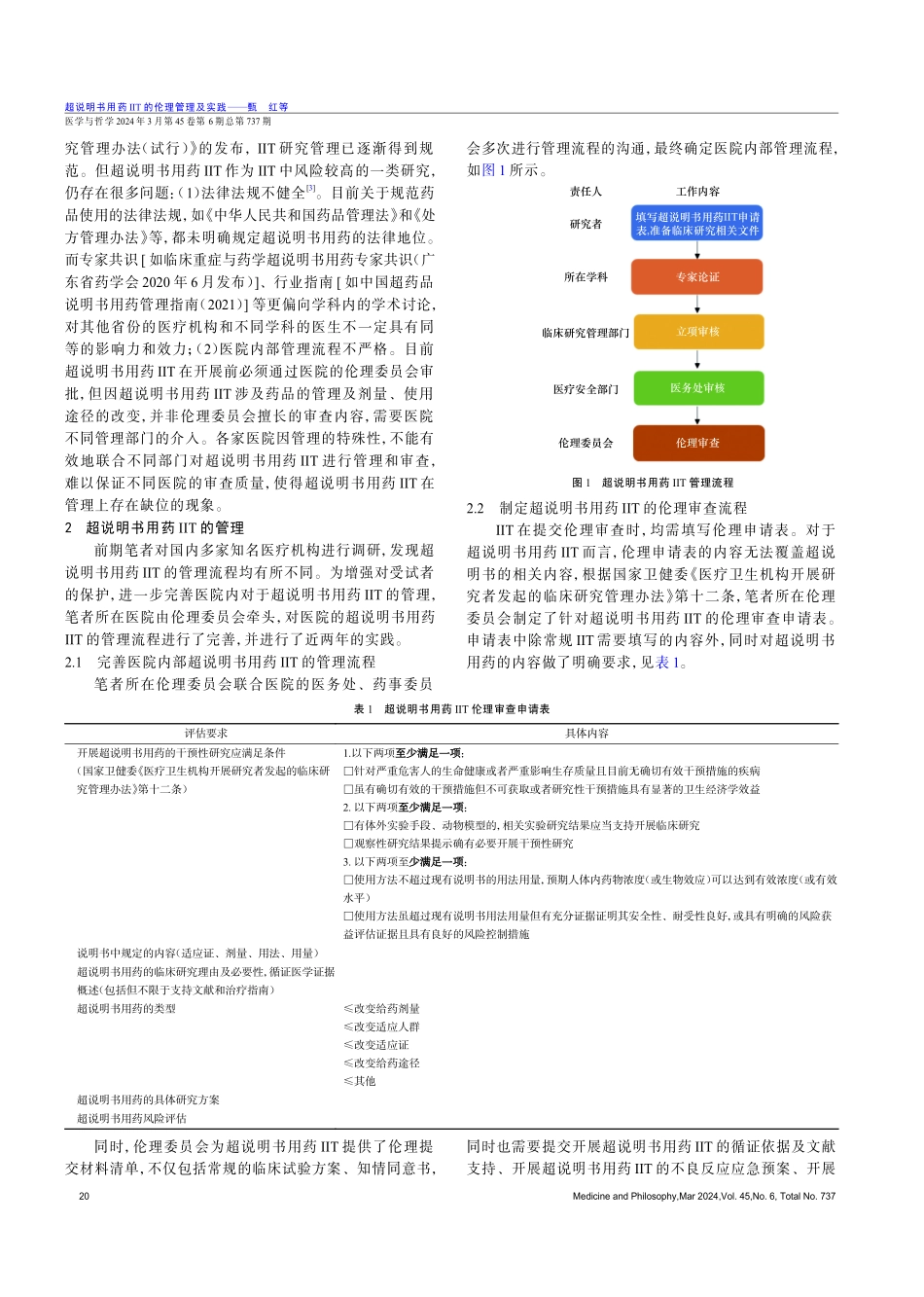
超说明书用药IIT的伦理管理及实践*甄红1,2刘墨池1,2王旭东1,2张峻1,2许锋1,2,3摘要:为规范研究者发起的超说明书用药临床研究管理,作者以所在医院实际情况为例,通过强化学科专家论证,结合药事委员会和医务处管理,完善医院内部管理流程,规范超说明书用药IIT的开展。结合超说明书用药IIT管理过程中所面临的关于立法、监管等方面存在的问题,提出管理建议。强化研究者对超说明书用药IIT的伦理意识、严格监督和管理,完善医疗机构内部管理流程,切实做好知情同意,保障受试者权益,同时鼓励研究者与药企开展实质性的超说明书用药IIT合作,合理平摊风险,以期为医疗机构的超说明书用药IIT管理提供建议,优化超说明书用药的管理现状。关键词:超说明书用药,临床研究,伦理审查中图分类号:R-05文献标识码:A文章编号:1002-0772(2024)06-0019-04DOI:10.12014/j.issn.1002-0772.2024.06.05EthicalManagementandPracticeofIITwithOff-labelDrugUseZHENHong1,2,LIUMochi1,2,WANGXudong1,2,ZHANGJun1,2,XUFeng1,2,3.1.InstitutionalReviewBoard,ShanghaiNinthPeoplesHospital,ShanghaiJiaoTongUniversitySchoolofMedicine,Shanghai200011,China;2.CenterforSpecialtyStrategyResearchofShanghaiJiaoTongUniversityChinaHospitalDevelopmentInstitute,Shanghai200011,China;3.DisciplinePlanningDepartment,ShanghaiNinthPeoplesHospital,ShanghaiJiaoTongUniver-sitySchoolofMedicineShanghai200011,ChinaAbstract:Tostandardizethemanagementofinvestigator-initiatedtrials(IITs)involvingoff-labeldruguse,theauthorsusetheactualsituationoftheirhospitalasanexample.Bystrengtheningdiscipline-specificexpertconsultation,integratingthemanagementofthePharmacyandTherapeuticsCommitteeandtheMedicalAffairsOffice,andrefininginternalmanagementprocesses,theauthorsenhancetheconductofIITsinvolvingoff-labeldruguse.ConsideringthechallengesinlegislationandregulationfacedduringthemanagementofIITswithoff-labeldruguse,theauthorsprovidemanagementrecommendations.Emphasisisplacedonresearchers'ethicalawareness,strictsupervision,andmanagementofIITsinvolvingoff-labeldruguse,improvinginternalmanagementprocessesofmedicalinstitutions,ensuringinformedconsent,andsafeguardingtherightsofparticipants.Additionally,res...