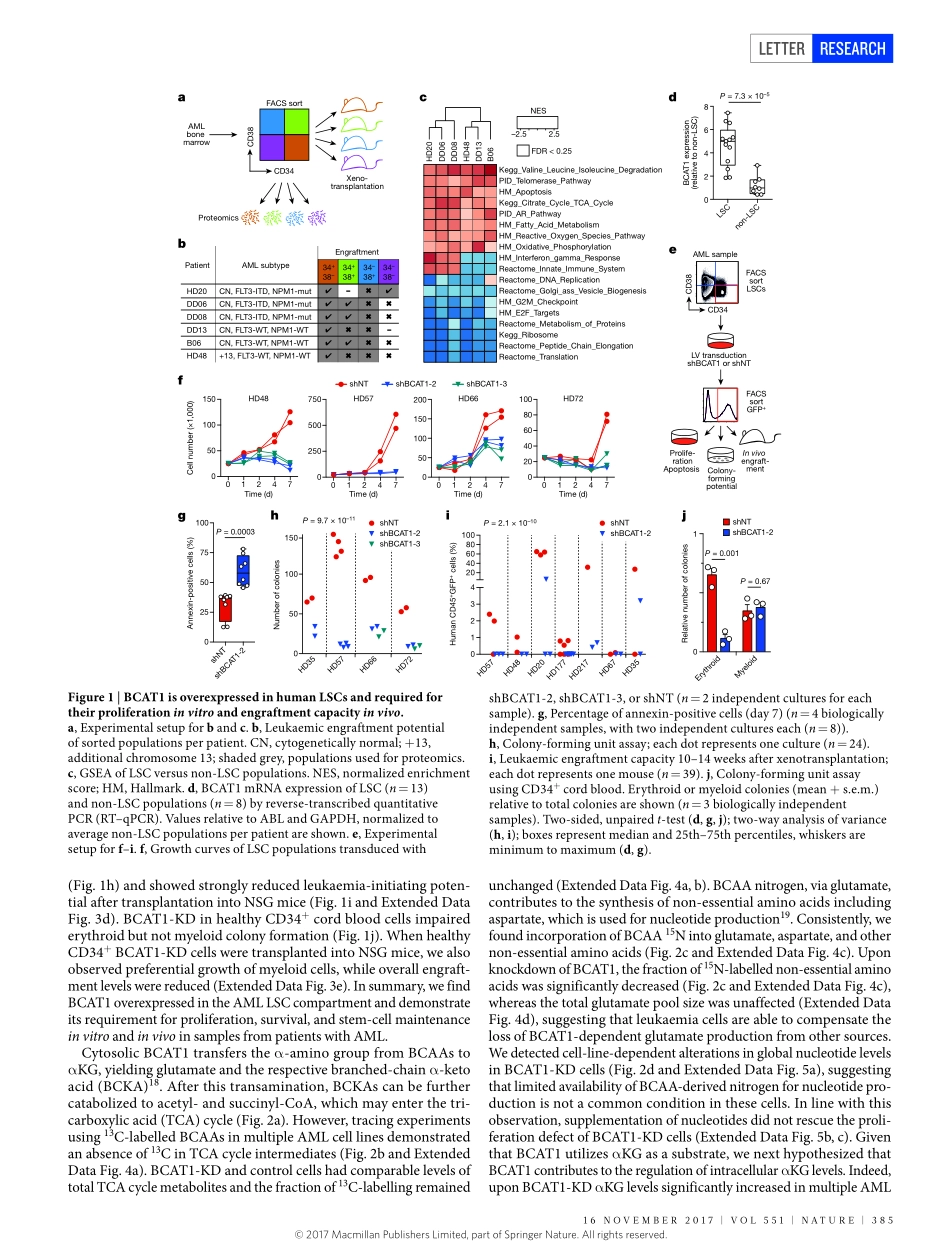
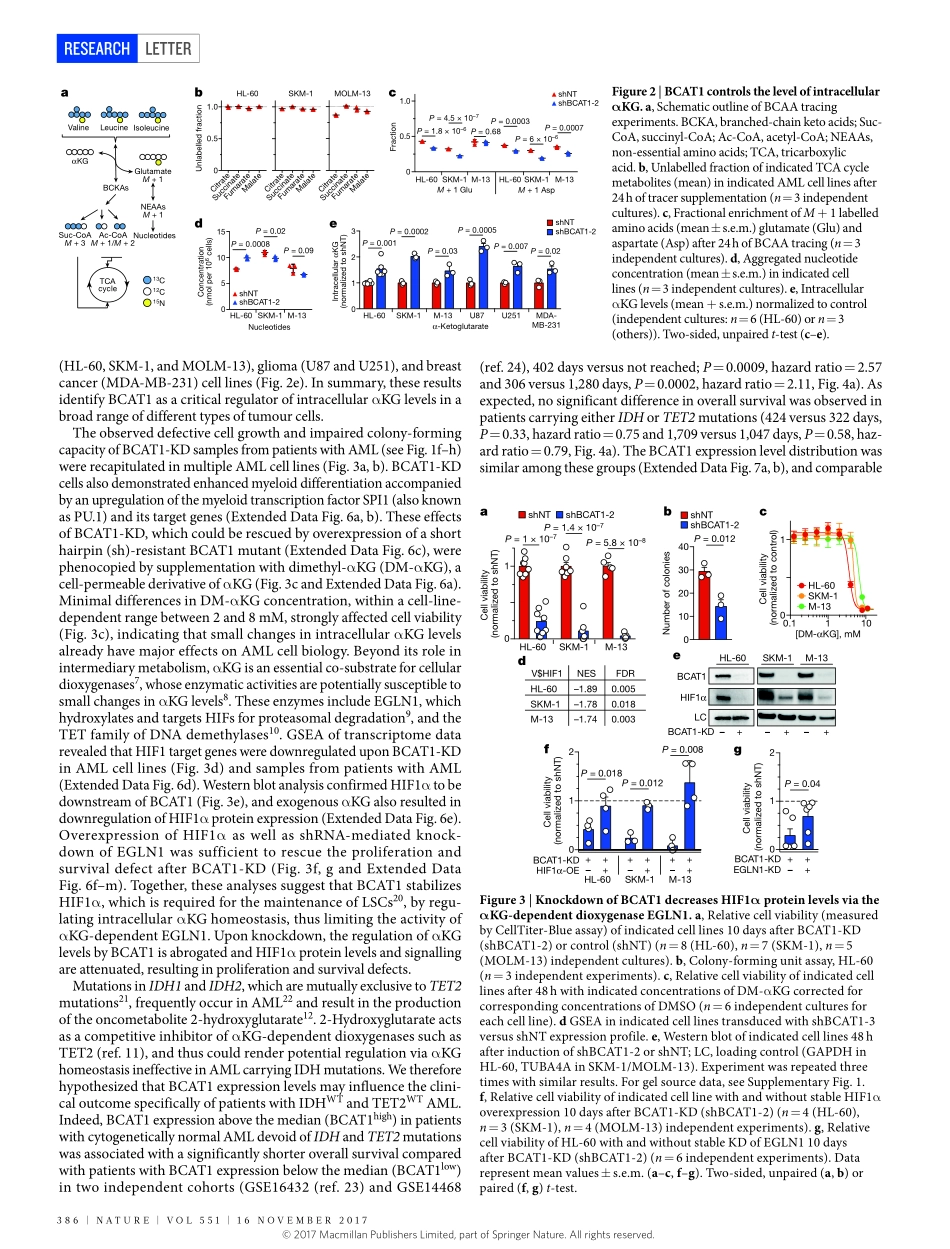
384|NATURE|VOL551|16NOVEmbER2017LETTERdoi:10.1038/nature24294BCAT1restrictsαKGlevelsinAMLstemcellsleadingtoIDHmut-likeDNAhypermethylationSimonRaffel1,2,3*,mattiaFalcone1,2*,NiclasKneisel4*,JennyHansson5,WeiWang4,ChristophLutz3,Larsbullinger6,GernotPoschet7,YannicNonnenmacher8,9,Andreabarnert1,2,Carstenbahr1,2,PetraZeisberger1,2,AdrianaPrzybylla1,2,markusSohn1,2,martjeTönjes4,AyeletErez10,LitalAdler10,PatriziaJensen11,ClaudiaScholl12,19,StefanFröhling11,13,19,SibylleCocciardi6,PatrickWuchter3,14,ChristianThiede15,AnneFlörcken16,JörgWestermann16,GerhardEhninger15,PeterLichter4,19,KarstenHiller8,9,RüdigerHell7,CarlHerrmann17,18,AnthonyD.Ho3,JeroenKrijgsveld5,bernhardRadlwimmer4,19§&AndreasTrumpp1,2,19§Thebranched-chainaminoacid(BCAA)pathwayandhighlevelsofBCAAtransaminase1(BCAT1)haverecentlybeenassociatedwithaggressivenessinseveralcancerentities1–6.However,themechanisticroleofBCAT1inthisprocessremainslargelyuncertain.Here,byperforminghigh-resolutionproteomicanalysisofhumanacutemyeloidleukaemia(AML)stem-cellandnon-stem-cellpopulations,wefindtheBCAApathwayenrichedandBCAT1proteinandtranscriptsoverexpressedinleukaemiastemcells.WeshowthatBCAT1,whichtransfersα-aminogroupsfromBCAAstoα-ketoglutarate(αKG),isacriticalregulatorofintracellularαKGhomeostasis.Furthertoitsroleinthetricarboxylicacidcycle,αKGisanessentialcofactorforαKG-dependentdioxygenasessuchasEgl-9familyhypoxiainduciblefactor1(EGLN1)andtheten-eleventranslocation(TET)familyofDNAdemethylases7–10.KnockdownofBCAT1inleukaemiacellscausedaccumulationofαKG,leadingtoEGLN1-mediatedHIF1αproteindegradation.Thisresultedinagrowthandsurvivaldefectandabrogatedleukaemia-initiatingpotential.Bycontrast,overexpressionofBCAT1inleukaemiacellsdecreasedintracellularαKGlevelsandcausedDNAhypermethylationthroughalteredTETactivity.AMLwithhighlevelsofBCAT1(BCAT1high)displayedaDNAhypermethylationphenotypesimilartocasescarryingamutantisocitratedehydrogenase(IDHmut),inwhichTET2isinhibitedbytheoncometabolite2-hydroxyglutarate11...