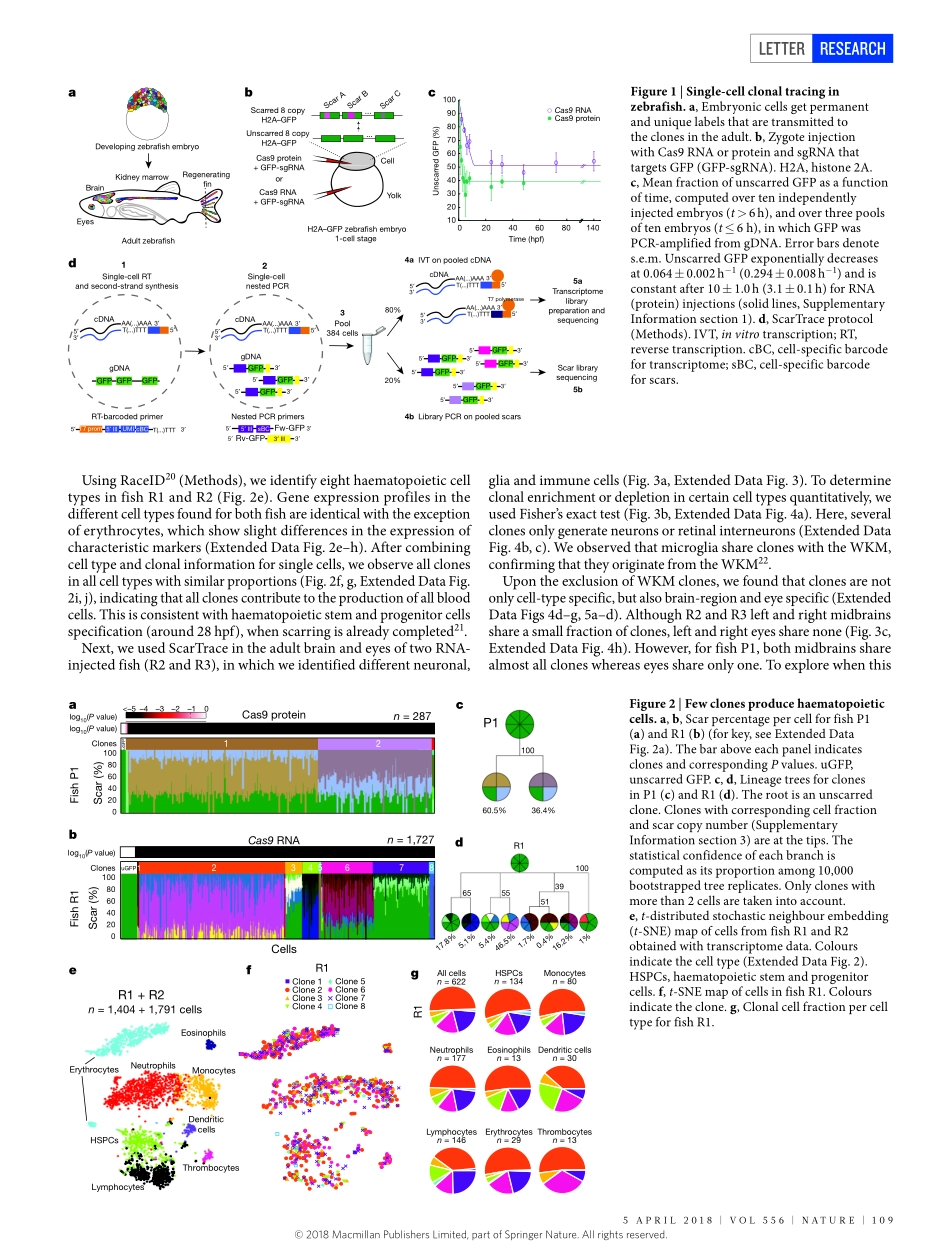
108|NATURE|VOL556|5ApRiL2018LETTERdoi:10.1038/nature25969Whole-organismclonetracingusingsingle-cellsequencingAnnaAlemany1*,MariaFlorescu1*,ChloéS.Baron1*,Josipeterson-Maduro1*&AlexandervanOudenaarden1Embryonicdevelopmentisacrucialperiodinthelifeofamulticellularorganism,duringwhichlimitedsetsofembryonicprogenitorsproduceallcellsintheadultbody.Determiningwhichfatetheseprogenitorsacquireinadulttissuesrequiresthesimultaneousmeasurementofclonalhistoryandcellidentityatsingle-cellresolution,whichhasbeenamajorchallenge.Clonalhistoryhastraditionallybeeninvestigatedbymicroscopicallytrackingcellsduringdevelopment1,2,monitoringtheheritableexpressionofgeneticallyencodedfluorescentproteins3and,morerecently,usingnext-generationsequencingtechnologiesthatexploitsomaticmutations4,microsatelliteinstability5,transposontagging6,viralbarcoding7,CRISPR–Cas9genomeediting8–13andCre–loxPrecombination14.Single-celltranscriptomics15providesapowerfulplatformforunbiasedcell-typeclassification.HerewepresentScarTrace,asingle-cellsequencingstrategythatenablesthesimultaneousquantificationofclonalhistoryandcelltypeforthousandsofcellsobtainedfromdifferentorgansoftheadultzebrafish.UsingScarTrace,weshowthatasmallsetofmultipotentembryonicprogenitorsgenerateallhaematopoieticcellsinthekidneymarrow,andthatmanyprogenitorsproducespecificcelltypesintheeyesandbrain.Inaddition,westudywhenembryonicprogenitorscommittotheleftorrighteye.ScarTracerevealsthatepidermalandmesenchymalcellsinthecaudalfinarisefromthesameprogenitors,andthatosteoblast-restrictedprecursorscanproducemesenchymalcellsduringregeneration.Furthermore,weidentifyresidentimmunecellsinthefinwithadistinctclonaloriginfromotherbloodcelltypes.Weenvisionthatsimilarapproacheswillhavemajorapplicationsinotherexperimentalsystems,inwhichthematchingofembryonicclonalorigintoadultcelltypewillultimatelyallowreconstructionofhowtheadultbodyisbuiltfromasinglecell.Thegoalofourexperimentistwofold:first,tolinkcellsintheembryototheircorrespondingclonesinadulttissue(...