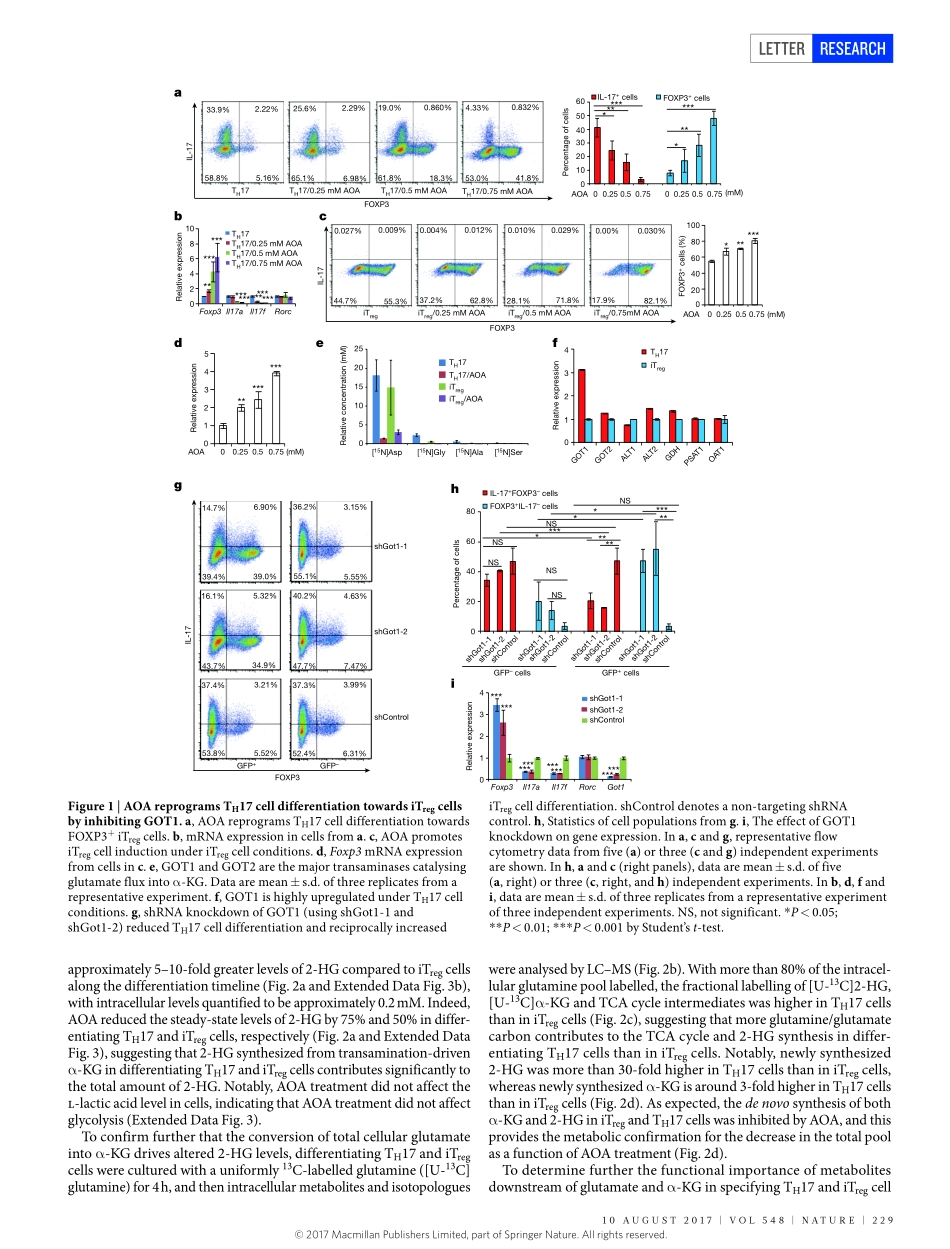
228|NATURE|VOL548|10AUgUsT2017LETTERdoi:10.1038/nature23475MetaboliccontrolofTH17andinducedTregcellbalancebyanepigeneticmechanismTaoXu1*,KellyM.stewart2*,XiaohuWang3*,KaiLiu1,MinXie1,JaeKyuRyu1,KeLi1,TianhuaMa1,4,HaixiaWang1,LuNi3,saiyongZhu1,NanCao1,DongweiZhu2,YuZhang1,KaterinaAkassoglou1,5,ChenDong3§,EdwardM.Driggers2†§&shengDing1,4§Metabolismhasbeenshowntointegratewithepigeneticsandtranscriptiontomodulatecellfateandfunction1–3.BeyondmeetingthebioenergeticandbiosyntheticdemandsofT-celldifferentiation4–8,whethermetabolismmightcontrolT-cellfatebyanepigeneticmechanismisunclear.Here,throughthediscoveryandmechanisticcharacterizationofasmallmolecule,(aminooxy)aceticacid,thatreprogramsthedifferentiationofThelper17(TH17)cellstowardsinducedregulatoryT(iTreg)cells,weshowthatincreasedtransamination,mainlycatalysedbyGOT1,leadstoincreasedlevelsof2-hydroxyglutarateindifferentiatingTH17cells.Theaccumulationof2-hydroxyglutarateresultedinhypermethylationoftheFoxp3genelocusandinhibitedFoxp3transcription,whichisessentialforfatedeterminationtowardsTH17cells.Inhibitionoftheconversionofglutamatetoα-ketoglutaricacidpreventedtheproductionof2-hydroxyglutarate,reducedmethylationoftheFoxp3genelocus,andincreasedFoxp3expression.ThisconsequentlyblockedthedifferentiationofTH17cellsbyantagonizingthefunctionoftranscriptionfactorRORγtandpromotedpolarizationintoiTregcells.SelectiveinhibitionofGOT1with(aminooxy)aceticacidamelioratedexperimentalautoimmuneencephalomyelitisinatherapeuticmousemodelbyregulatingthebalancebetweenTH17andiTregcells.Targetingaglutamate-dependentmetabolicpathwaythusrepresentsanewstrategyfordevelopingtherapeuticagentsagainstTH17-mediatedautoimmunediseases.ToidentifysmallmoleculesthatreprogramTH17differentiationtowardsiTregcellfate,wescreened10,000smallmoleculesusingCD4+naiveTcellsfromIL-17F–RFP/FOXP3–GFPmiceculturedunderoptimalTH17differentiationconditions9(ExtendedDataFig.1a).Asmallmolecule,(aminooxy)aceticacid(AOA),wasfoundtorepro-gramTH17inductiontoiTregcellsin...