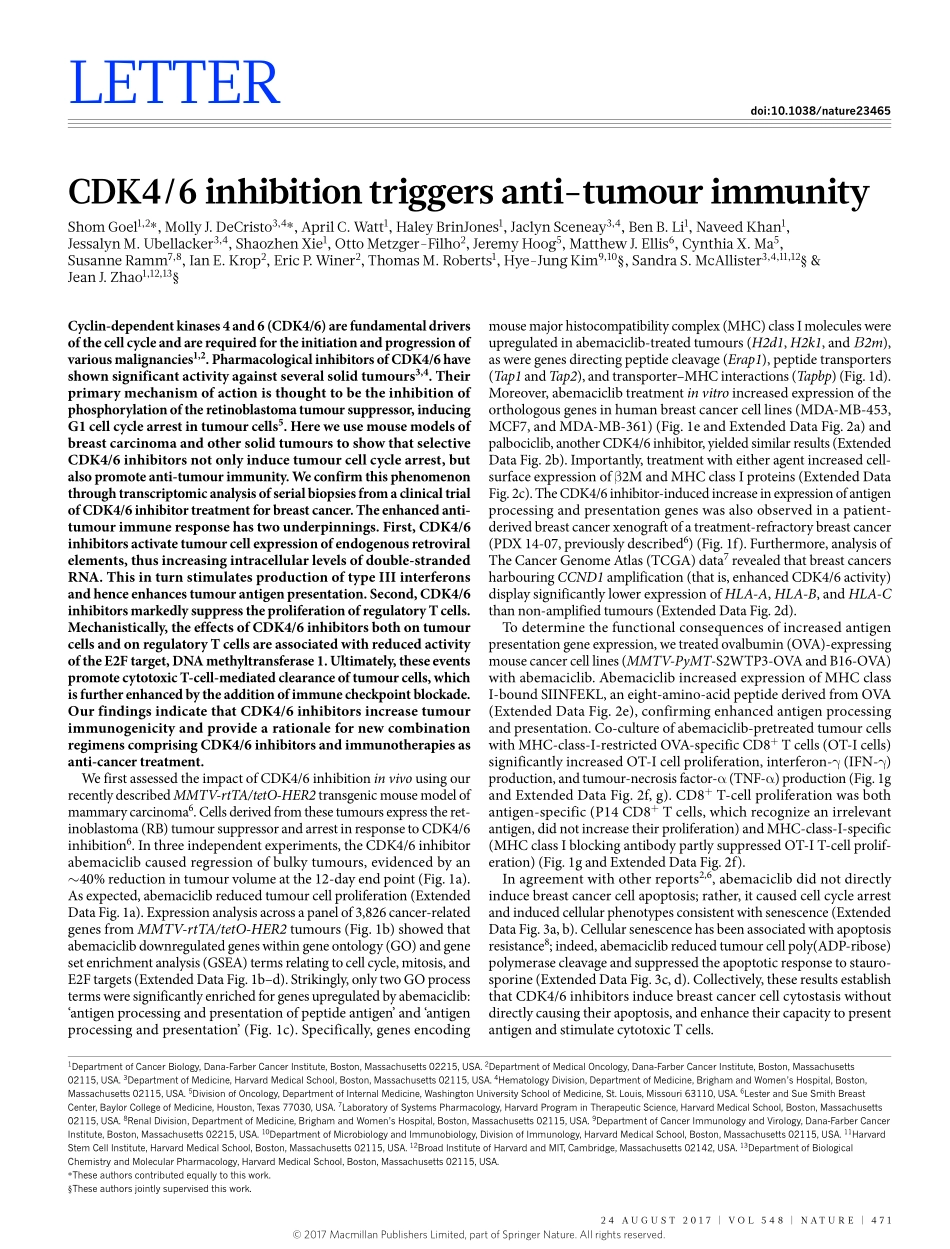
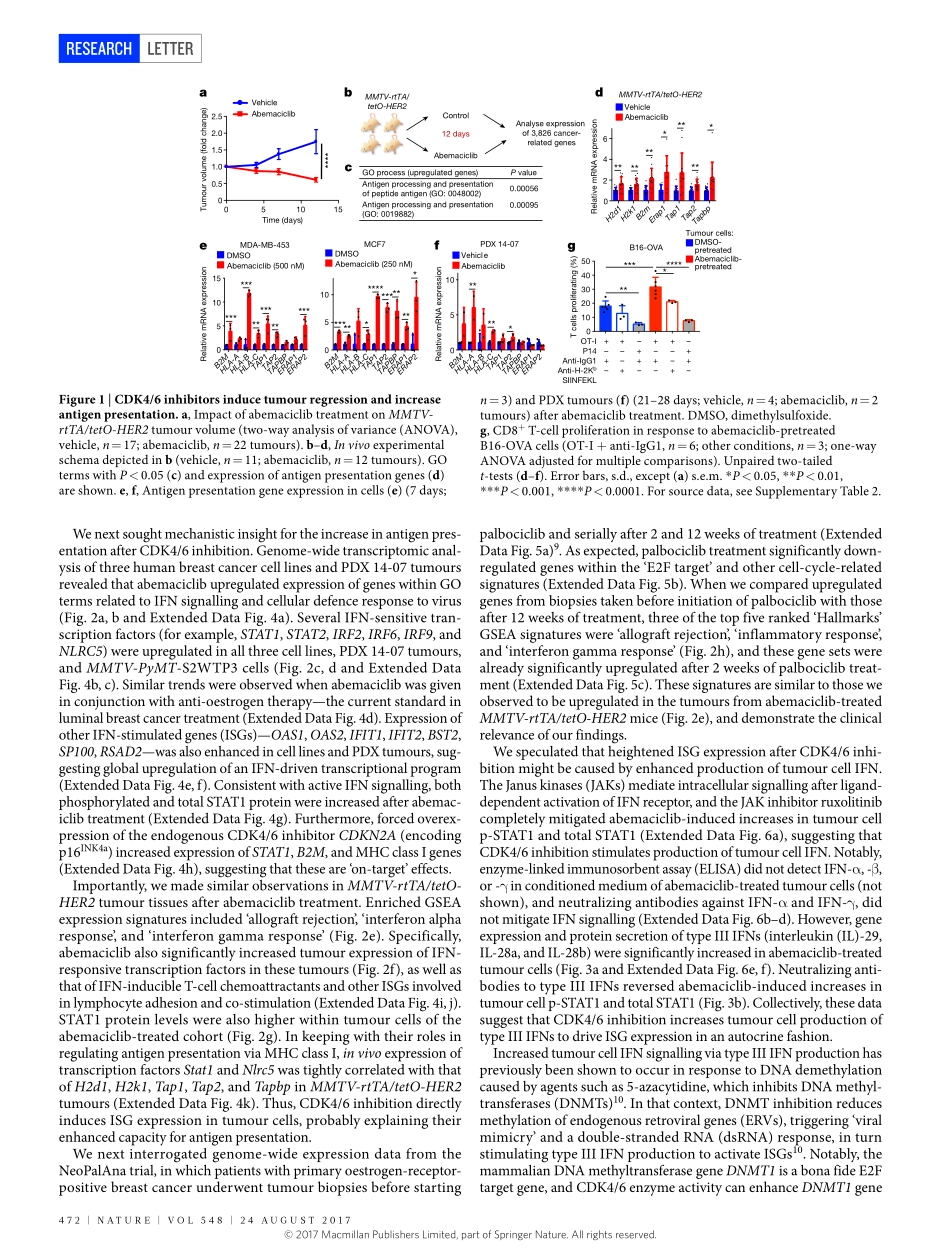
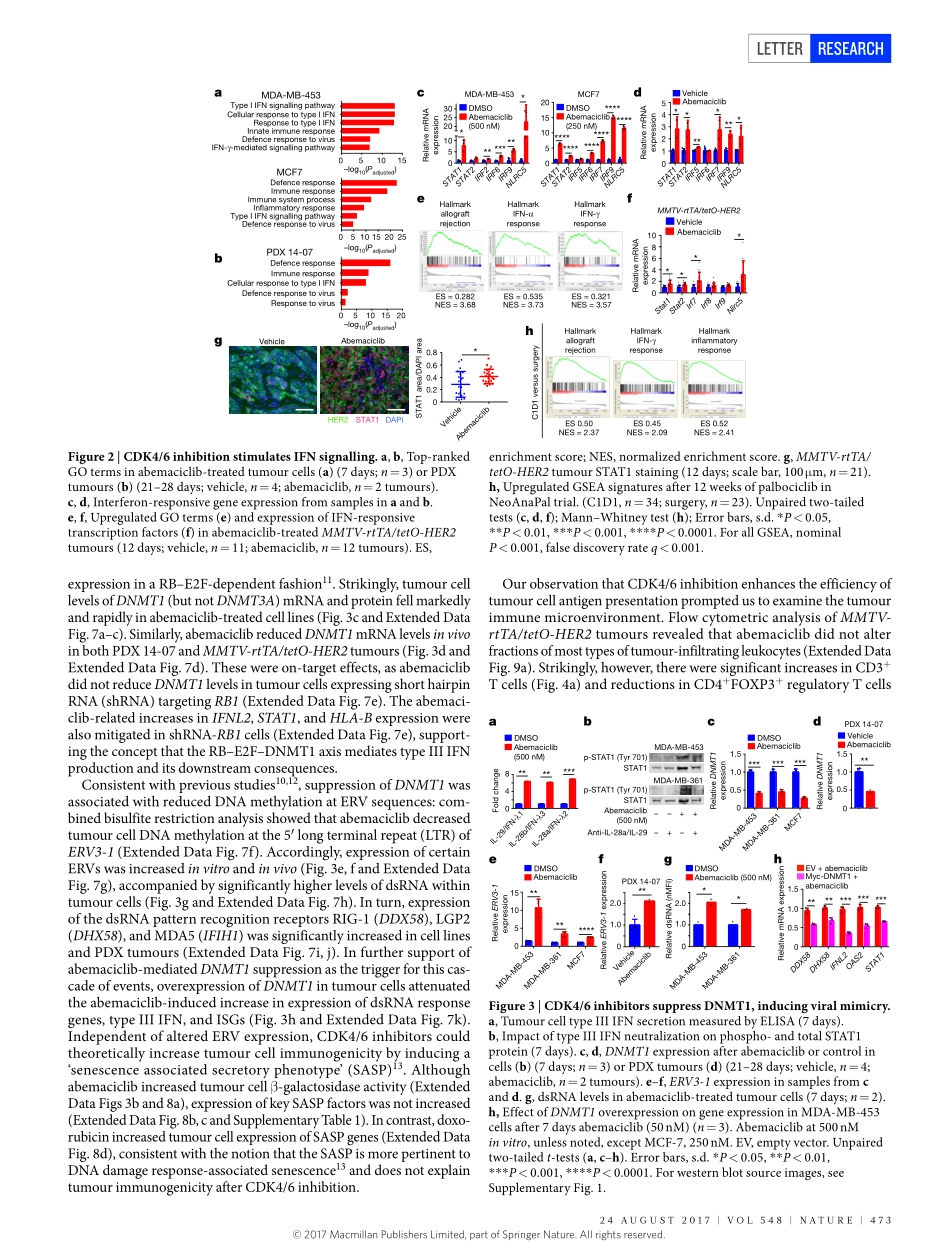
24august2017|VOL548|NatuRE|471LEttERdoi:10.1038/nature23465CDK4/6inhibitiontriggersanti-tumourimmunityshomgoel1,2*,MollyJ.DeCristo3,4*,aprilC.Watt1,HaleyBrinJones1,Jaclynsceneay3,4,BenB.Li1,NaveedKhan1,JessalynM.ubellacker3,4,shaozhenXie1,OttoMetzger-Filho2,JeremyHoog5,MatthewJ.Ellis6,CynthiaX.Ma5,susanneRamm7,8,IanE.Krop2,EricP.Winer2,thomasM.Roberts1,Hye-JungKim9,10§,sandras.Mcallister3,4,11,12§&JeanJ.Zhao1,12,13§Cyclin-dependentkinases4and6(CDK4/6)arefundamentaldriversofthecellcycleandarerequiredfortheinitiationandprogressionofvariousmalignancies1,2.PharmacologicalinhibitorsofCDK4/6haveshownsignificantactivityagainstseveralsolidtumours3,4.Theirprimarymechanismofactionisthoughttobetheinhibitionofphosphorylationoftheretinoblastomatumoursuppressor,inducingG1cellcyclearrestintumourcells5.HereweusemousemodelsofbreastcarcinomaandothersolidtumourstoshowthatselectiveCDK4/6inhibitorsnotonlyinducetumourcellcyclearrest,butalsopromoteanti-tumourimmunity.WeconfirmthisphenomenonthroughtranscriptomicanalysisofserialbiopsiesfromaclinicaltrialofCDK4/6inhibitortreatmentforbreastcancer.Theenhancedanti-tumourimmuneresponsehastwounderpinnings.First,CDK4/6inhibitorsactivatetumourcellexpressionofendogenousretroviralelements,thusincreasingintracellularlevelsofdouble-strandedRNA.ThisinturnstimulatesproductionoftypeIIIinterferonsandhenceenhancestumourantigenpresentation.Second,CDK4/6inhibitorsmarkedlysuppresstheproliferationofregulatoryTcells.Mechanistically,theeffectsofCDK4/6inhibitorsbothontumourcellsandonregulatoryTcellsareassociatedwithreducedactivityoftheE2Ftarget,DNAmethyltransferase1.Ultimately,theseeventspromotecytotoxicT-cell-mediatedclearanceoftumourcells,whichisfurtherenhancedbytheadditionofimmunecheckpointblockade.OurfindingsindicatethatCDK4/6inhibitorsincreasetumourimmunogenicityandprovidearationalefornewcombinationregimenscomprisingCDK4/6inhibitorsandimmunotherapiesasanti-cancertreatment.WefirstassessedtheimpactofCDK4/6inhibitioninvivousingourrecentlydescribedMMTV-rtTA...