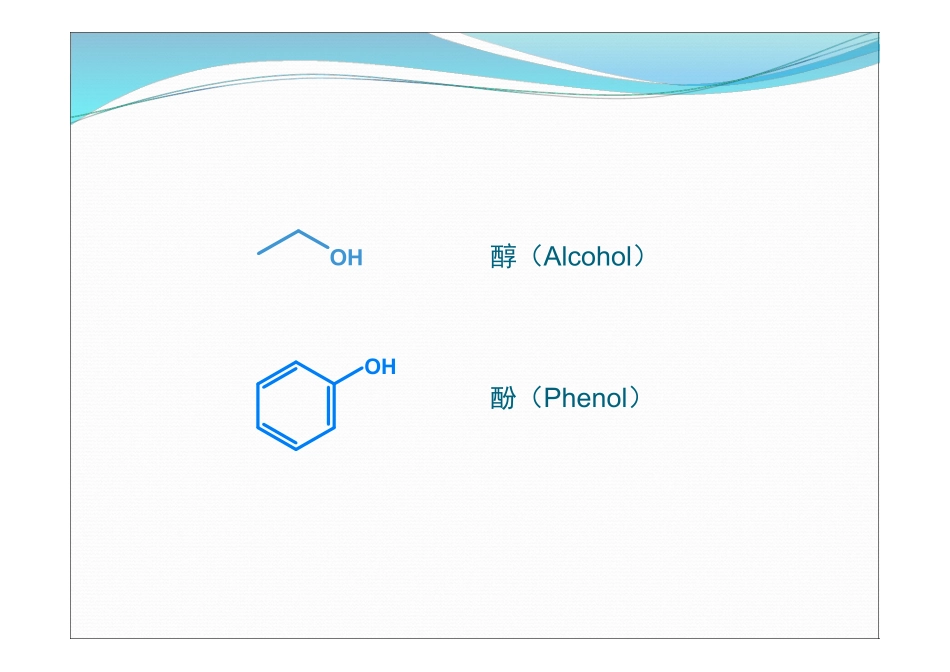
Alcoholsandphenols第十章醇与酚OrganicChemistryA(1)ByProf.LiYan-MeiTsinghuaUniversityOHOHOHHOOHHOOH醇(Alcohol)酚(Phenol)ContentContentContentPartIAlcohols10.1Structure,isomerization,classificationandnomenclature10.2Physicalandspectroscopicproperties10.3Monobasic/Monohydroxylalcohol10.4Polybasicalcohol10.5Preparationofalcohol10.6SourcesandusagesPartIIPhenol/Hydroxybenzene10.7Structureandnomenclature10.8Physicalandspectroscopicproperties10.9Chemicalreactions10.10Preparations10.11SourcesandusagesPartIPartIAlcoholsAlcohols第一部分第一部分醇醇10.1.1Structure10.1.2Isomerization10.1.3Classification10.1.4Nomenclature10.1Structure,isomerization,classificationandnomenclature108.5osp3hybridizationμ≈2DVeryclosetowaterManyalcoholscompletelymisciblewithwater10.1.1Structure结构OH10.1.2Isomerization异构PositionisomerizationOHOHOHOHOFuctionalgroupisomerizationCarbonchainisomerization位置异构碳架异构官能团异构10.1.3Classification分类C12OHOHHOOHHOOHOHOHOH根据羟基数目:一元醇二元醇三元醇根据所连的碳原子不同:一级醇(伯醇)二级醇(仲醇)三级醇(叔醇)根据所含碳原子数目:低级醇中级醇高级醇10.1.4Nomenclature命名OHDecidingthelongestchaintowhichthehydroxylgroupisattached1Decidetheserialnumberforallcarbonatoms.2Writedownthename.3OH123452-pentanolOH5,5-dimethyl-2-hexanolCH3OH5-methyl-3-hexanolCH3OH5-methylhex-4-en-2-olOH(E)-pent-3-en-1-olOHO2N4-nitrophenolIntermolecularhydrogenbondBoilingpointMeltingpointSimplealcoholscompletelymisciblewithwater与水形成氢键低级醇一般与水任意混溶OHHOHOOHHOHOHH10.2Physicalandspectroscopicproperties10.2.1Physicalproperties分子间氢键导致熔点、沸点较高Simplealcoholsmayformco-crystalswithinorganicsalts.低级醇与一些无机盐(如:MgCl2、CaCl2、CuSO4等)形成结晶状的分子化合物-结晶醇(醇化物)MgCl2,CuSO4……MgCl2·6CH3OHCaCl2·4C2H5OHCaCl2·4CH3OH用途:除去有机溶剂中少量的醇将醇与其它有机物分开危害:不可用MgCl2、CaCl2、CuSO4等来干燥低级醇10.2.1Spectrometryν(O-H)ν(C-O)3640cm-13630cm-1OHOHOH3620cm-11050cm-11100cm-11150cm-1IRν(O-H)3550~3450cm-1ν...