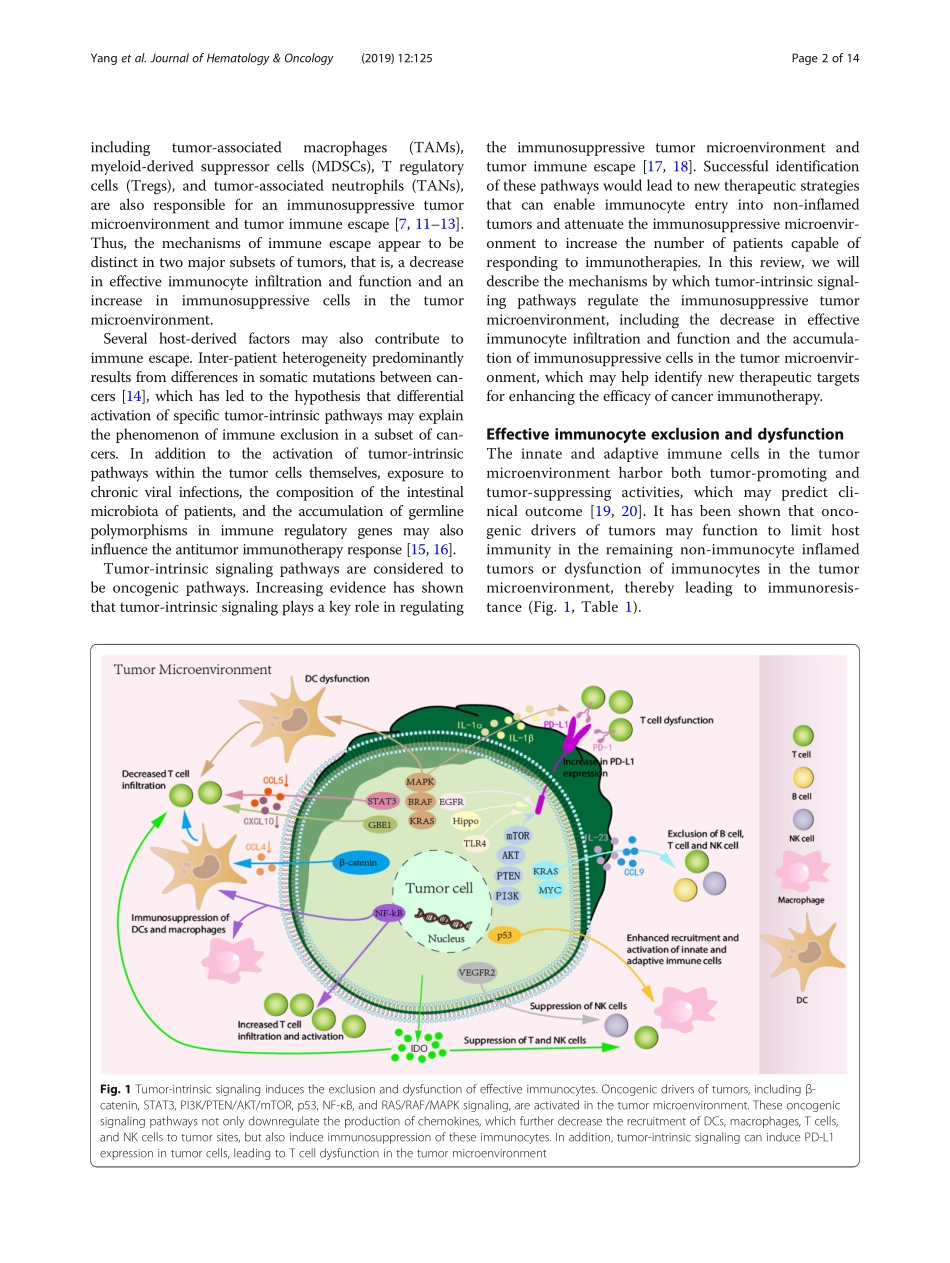
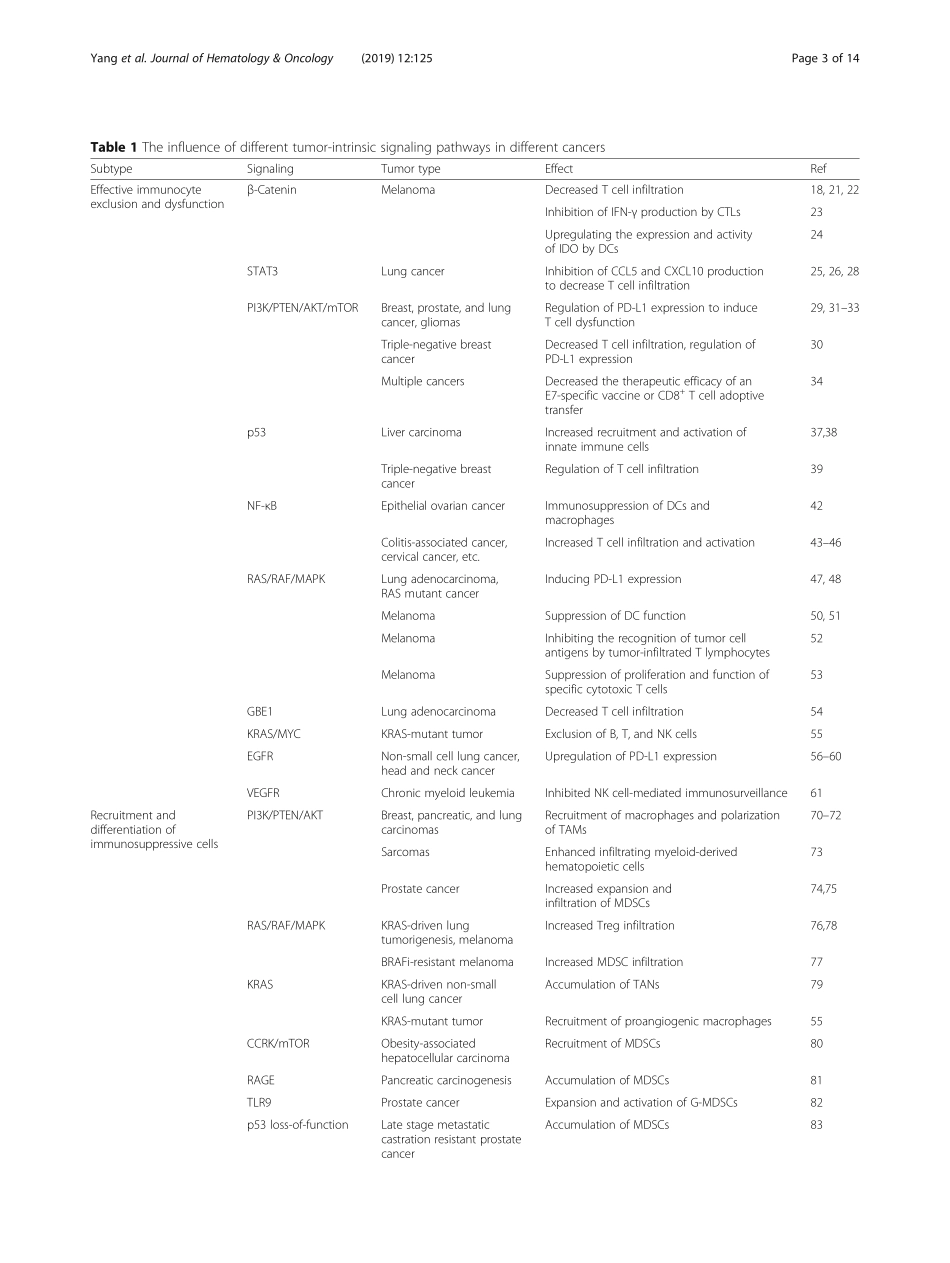
REVIEWOpenAccessTumor-intrinsicsignalingpathways:keyrolesintheregulationoftheimmunosuppressivetumormicroenvironmentLiYang1,2,4,AitianLi1,2,4,QingyangLei1,2,4andYiZhang1,2,3,4*AbstractImmunotherapyisacurrentlypopulartreatmentstrategyforcancerpatients.Althoughrecentdevelopmentsincancerimmunotherapyhavehadsignificantclinicalimpact,onlyasubsetofpatientsexhibitsclinicalresponse.Therefore,understandingthemolecularmechanismsofimmunotherapyresistanceisnecessary.Themechanismsofimmuneescapeappeartoconsistoftwodistincttumorcharacteristics:adecreaseineffectiveimmunocyteinfiltrationandfunctionandtheaccumulationofimmunosuppressivecellsinthetumormicroenvironment.Severalhost-derivedfactorsmayalsocontributetoimmuneescape.Moreover,inter-patientheterogeneitypredominantlyresultsfromdifferencesinsomaticmutationsbetweencancers,whichhasledtothehypothesisthatdifferentialactivationofspecifictumor-intrinsicpathwaysmayexplainthephenomenonofimmuneexclusioninasubsetofcancers.Increasingevidencehasalsoshownthattumor-intrinsicsignalingplaysakeyroleinregulatingtheimmunosuppressivetumormicroenvironmentandtumorimmuneescape.Therefore,understandingthemechanismsunderlyingimmuneavoidancemediatedbytumor-intrinsicsignalingmayhelpidentifynewtherapeutictargetsforexpandingtheefficacyofcancerimmunotherapies.Keywords:Immunosuppressivetumormicroenvironment,Immuneescape,Tcellinfiltration,Immunosuppressivecells,Tumor-intrinsicsignalingBackgroundTherecentdevelopmentsincancerimmunotherapyshowsignificantclinicalimpact.Particularly,monoclonalantibodiestargetingtheimmunecheckpointscytotoxicT-lymphocyte-associatedprotein4(CTLA-4)andprogrammedcelldeathprotein1(PD-1)haveshowndramaticefficacyandhavebeenapprovedbytheFDAforcancertreatment[1–4].Nevertheless,onlyasubsetofpatientsexperiencesclinicalbenefit.Furthermore,chimericantigenreceptor-T(CAR-T)celltherapyhasbeenapprovedforthetreatmentofcertainhematologicalmalignancies,yetsolidcancersareoftenlesssusceptibletoCAR-Tcelltherapymostlyduetotheimmunosuppressivetumormicroenvi...