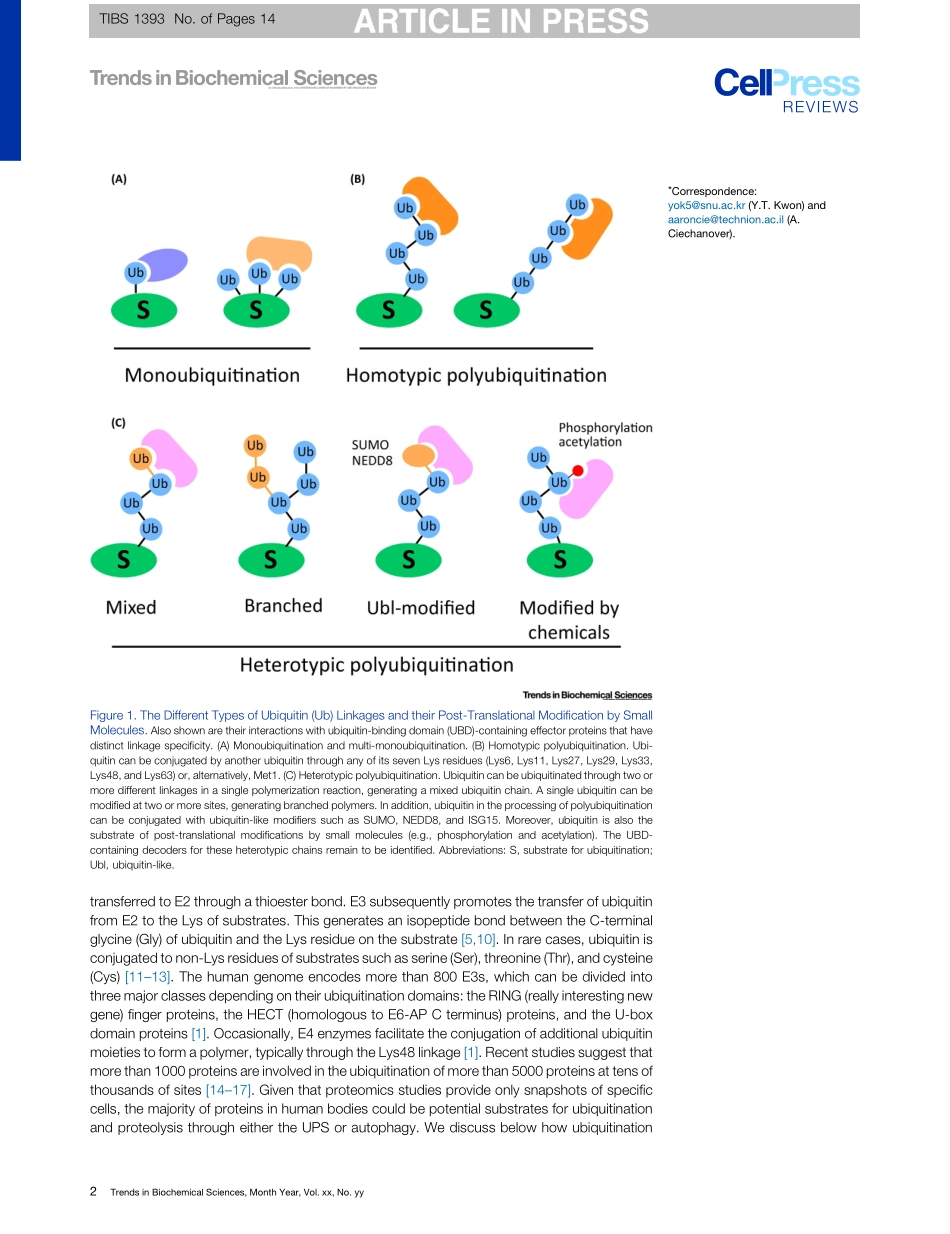
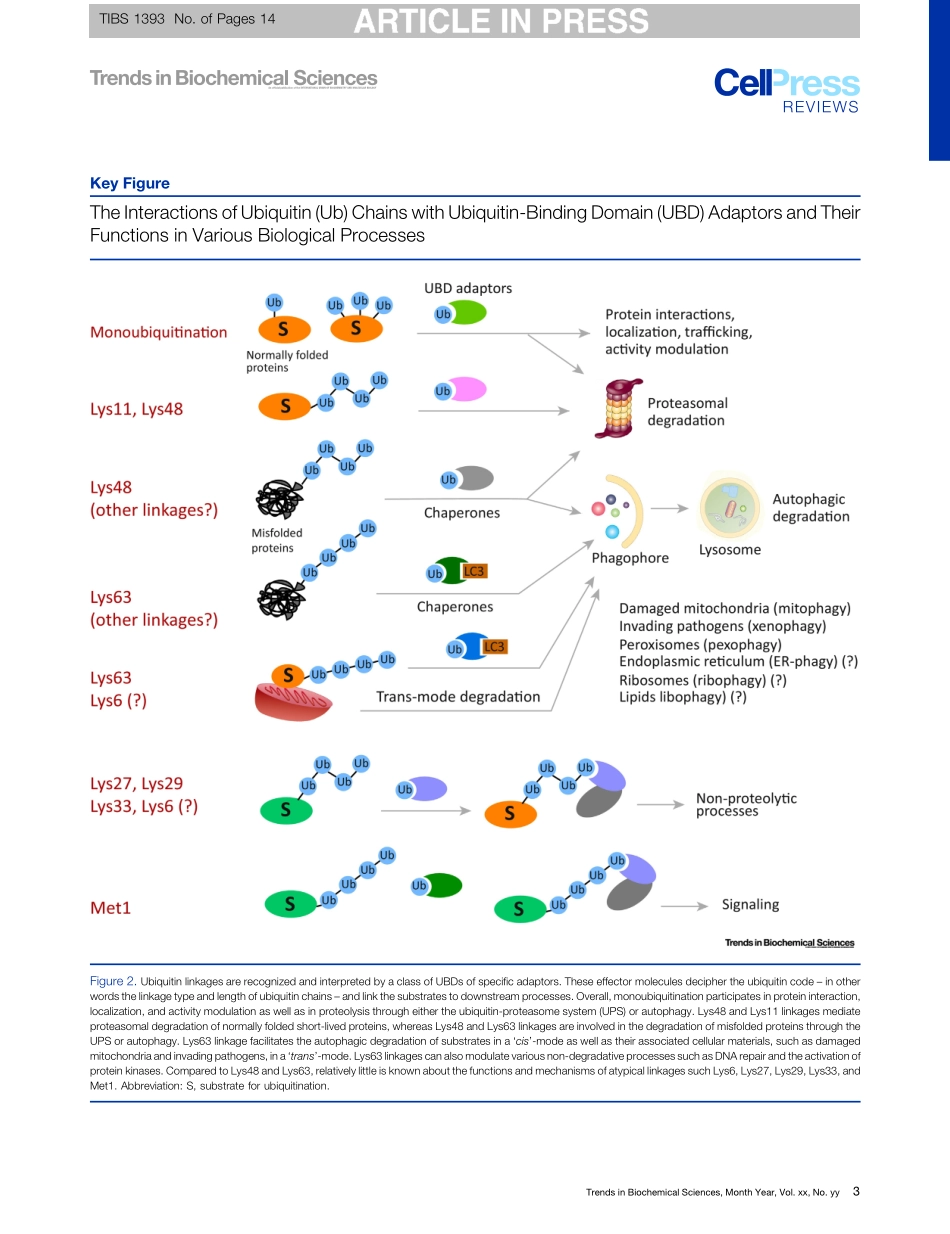
ReviewTheUbiquitinCodeintheUbiquitin-ProteasomeSystemandAutophagyYongTaeKwon1,2,*andAaronCiechanover1,3,*Theconjugationofthe76aminoacidproteinubiquitintootherproteinscanalterthemetabolicstabilityornon-proteolyticfunctionsofthesubstrate.Onceattachedtoasubstrate(monoubiquitination),ubiquitincanitselfbeubiquiti-natedonanyofitssevenlysine(Lys)residuesoritsN-terminalmethionine(Met1).Asingleubiquitinpolymermaycontainmixedlinkagesand/ortwoormorebranches.Inaddition,ubiquitincanbeconjugatedwithubiquitin-likemodifierssuchasSUMOorsmallmoleculessuchasphosphate.Thediversewaystoassembleubiquitinchainsprovidecountlessmeanstomodulatebiologicalprocesses.Weoverviewherethecomplexityoftheubiquitincode,withanemphasisontheemergingroleoflinkage-specificdegradationsignals(degrons)intheubiquitin-proteasomesystem(UPS)andtheautophagy-lyso-somesystem(hereafterautophagy).Ubiquitin-BasedDegronsinCellularDegradativePathwaysEukaryoticcellsoperatetwomajorproteolyticsystems:theUPSandautophagy.TheUPSisaselectiveproteolyticsysteminwhichtheconjugationofubiquitintosubstratesinducesdegradationbytheproteasome[1–3].Autophagyisaprocessbywhichcytoplasmiccon-stituentsaredegradedbythelysosome[4].Thisbulkdegradativesystemcanbedividedintomicroautophagy,chaperone-mediatedautophagy,andmacroautophagy,dependingonthemechanismofcargodeliverytothelysosome.TheUPSandautophagyarerespectivelyresponsibleforapproximately80–90%and10–20%ofcellularproteolysis,althoughtheratiosmayvarydependingonphysiologicalstatesandcelltypes[2,5,6].Centraltobothdegradativepathwaysisubiquitination,whichgenerateslinkage-specificdegronsonsubstratesdestinedfordestruction(Figure1).Ubiquitin-baseddegronsarerecognizedandboundbyaclassofubiquitin-bindingdomains(UBDs)ofspecificadaptors(Figure2,KeyFigure)[7,8].UBDsdecipherthepolyubiquitincode(i.e.,thelinkagetypeandlengthofubiquitinchains)andlinkthesubstratestodownstreamprocesses.SomeUBDadaptorsdeliverubiquitinatedsubstratestodegradativemachinery–theproteasome(e.g.,RAD23andUBQLNs)orautophagicvacuole...