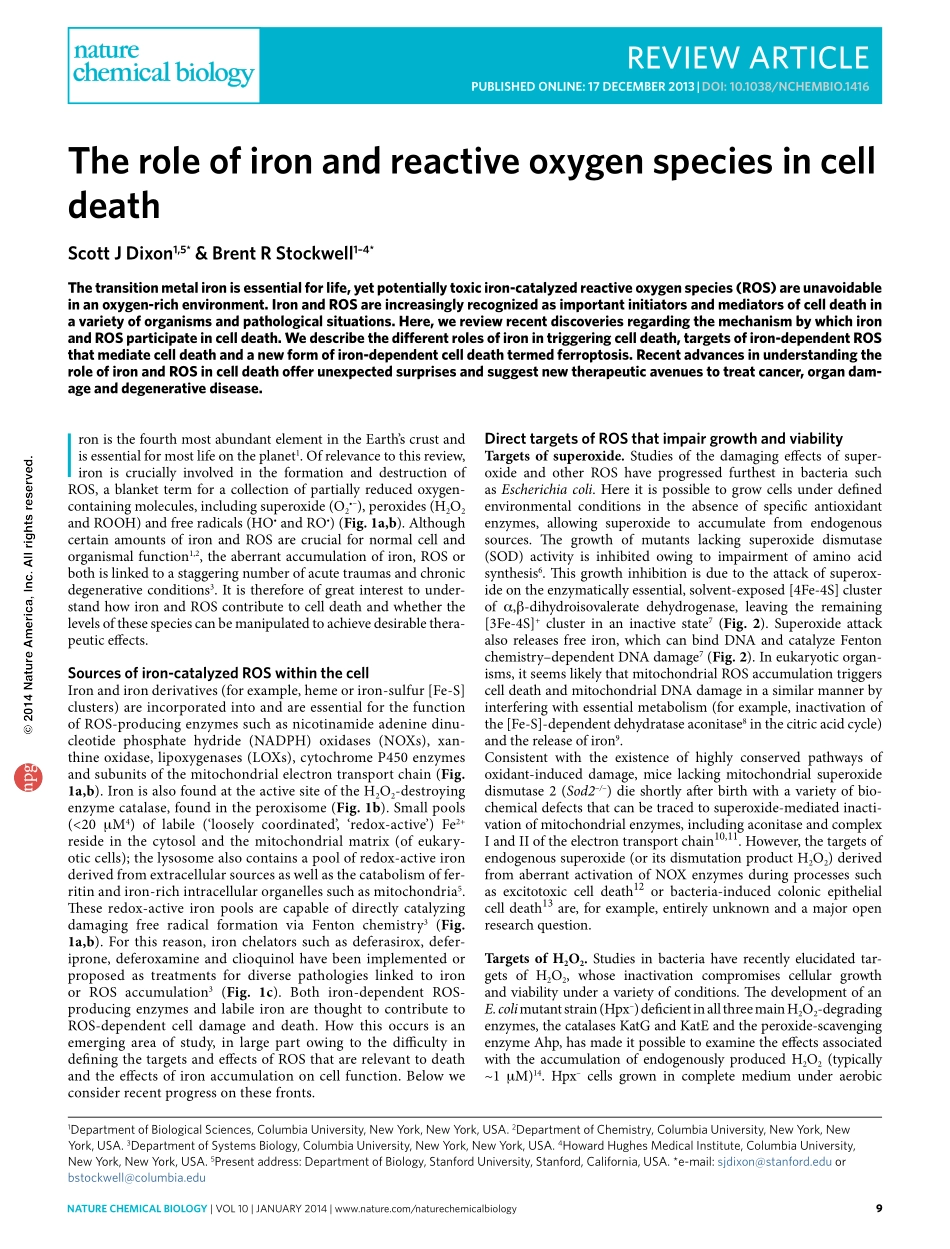
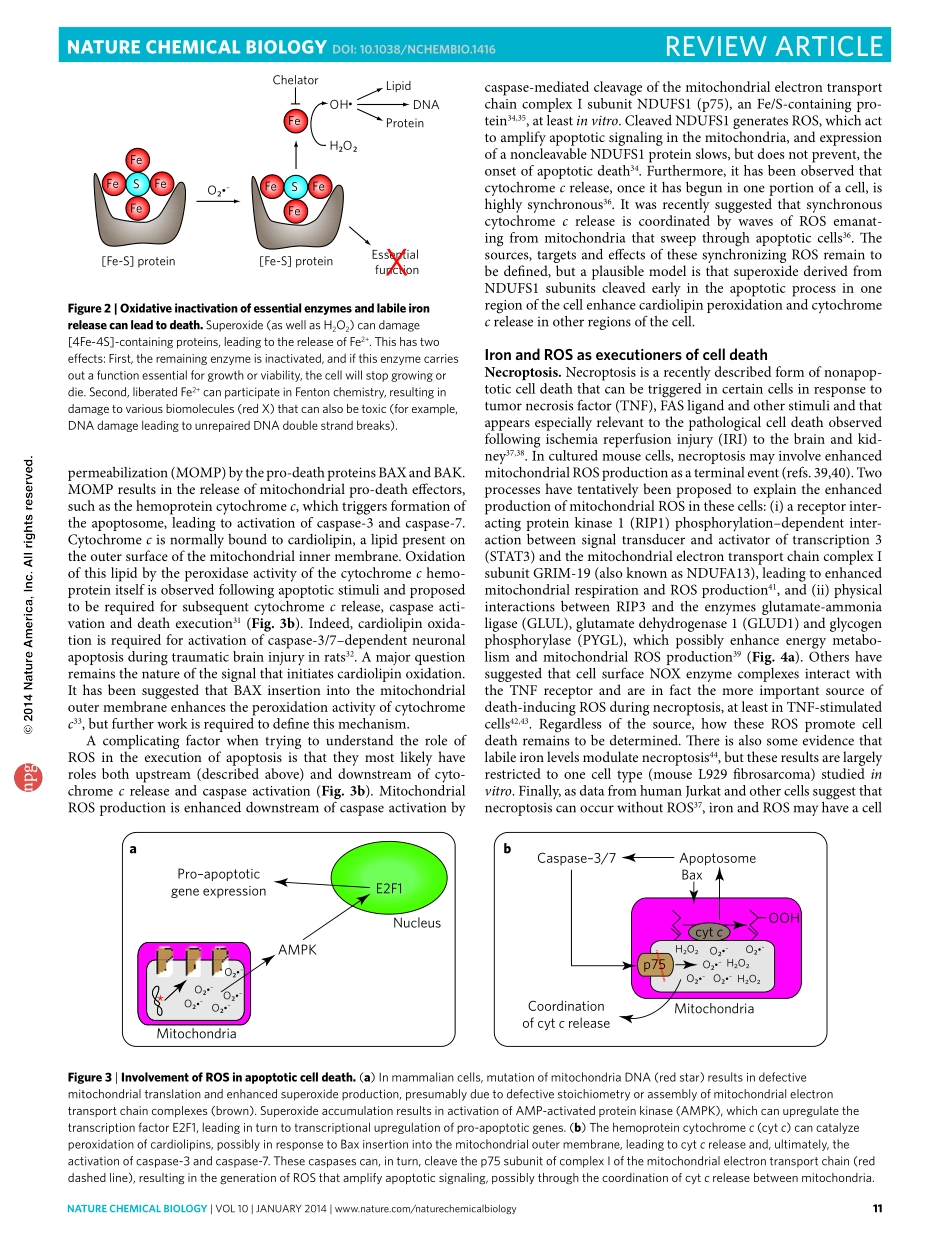
naturechemicalbiology|VOL10|JANUARY2014|www.nature.com/naturechemicalbiology9REVIEWARTICLEPublishedonline:17december2013|doi:10.1038/nchembio.1416IronisthefourthmostabundantelementintheEarth’scrustandisessentialformostlifeontheplanet1.Ofrelevancetothisreview,ironiscruciallyinvolvedintheformationanddestructionofROS,ablankettermforacollectionofpartiallyreducedoxygen-containingmolecules,includingsuperoxide(O2•–),peroxides(H2O2andROOH)andfreeradicals(HO•andRO•)(Fig.1a,b).AlthoughcertainamountsofironandROSarecrucialfornormalcellandorganismalfunction1,2,theaberrantaccumulationofiron,ROSorbothislinkedtoastaggeringnumberofacutetraumasandchronicdegenerativeconditions3.Itisthereforeofgreatinteresttounder-standhowironandROScontributetocelldeathandwhetherthelevelsofthesespeciescanbemanipulatedtoachievedesirablethera-peuticeffects.sourcesofiron-catalyzedroswithinthecellIronandironderivatives(forexample,hemeoriron-sulfur[Fe-S]clusters)areincorporatedintoandareessentialforthefunctionofROS-producingenzymessuchasnicotinamideadeninedinu-cleotidephosphatehydride(NADPH)oxidases(NOXs),xan-thineoxidase,lipoxygenases(LOXs),cytochromeP450enzymesandsubunitsofthemitochondrialelectrontransportchain(Fig.1a,b).IronisalsofoundattheactivesiteoftheH2O2-destroyingenzymecatalase,foundintheperoxisome(Fig.1b).Smallpools(<20mM4)oflabile(‘looselycoordinated’,‘redox-active’)Fe2+resideinthecytosolandthemitochondrialmatrix(ofeukary-oticcells);thelysosomealsocontainsapoolofredox-activeironderivedfromextracellularsourcesaswellasthecatabolismoffer-ritinandiron-richintracellularorganellessuchasmitochondria5.Theseredox-activeironpoolsarecapableofdirectlycatalyzingdamagingfreeradicalformationviaFentonchemistry3(Fig.1a,b).Forthisreason,ironchelatorssuchasdeferasirox,defer-iprone,deferoxamineandclioquinolhavebeenimplementedorproposedastreatmentsfordiversepathologieslinkedtoironorROSaccumulation3(Fig.1c).Bothiron-dependentROS-producingenzymesandlabileironarethoughttocontributetoROS-dependentcelldamagean...