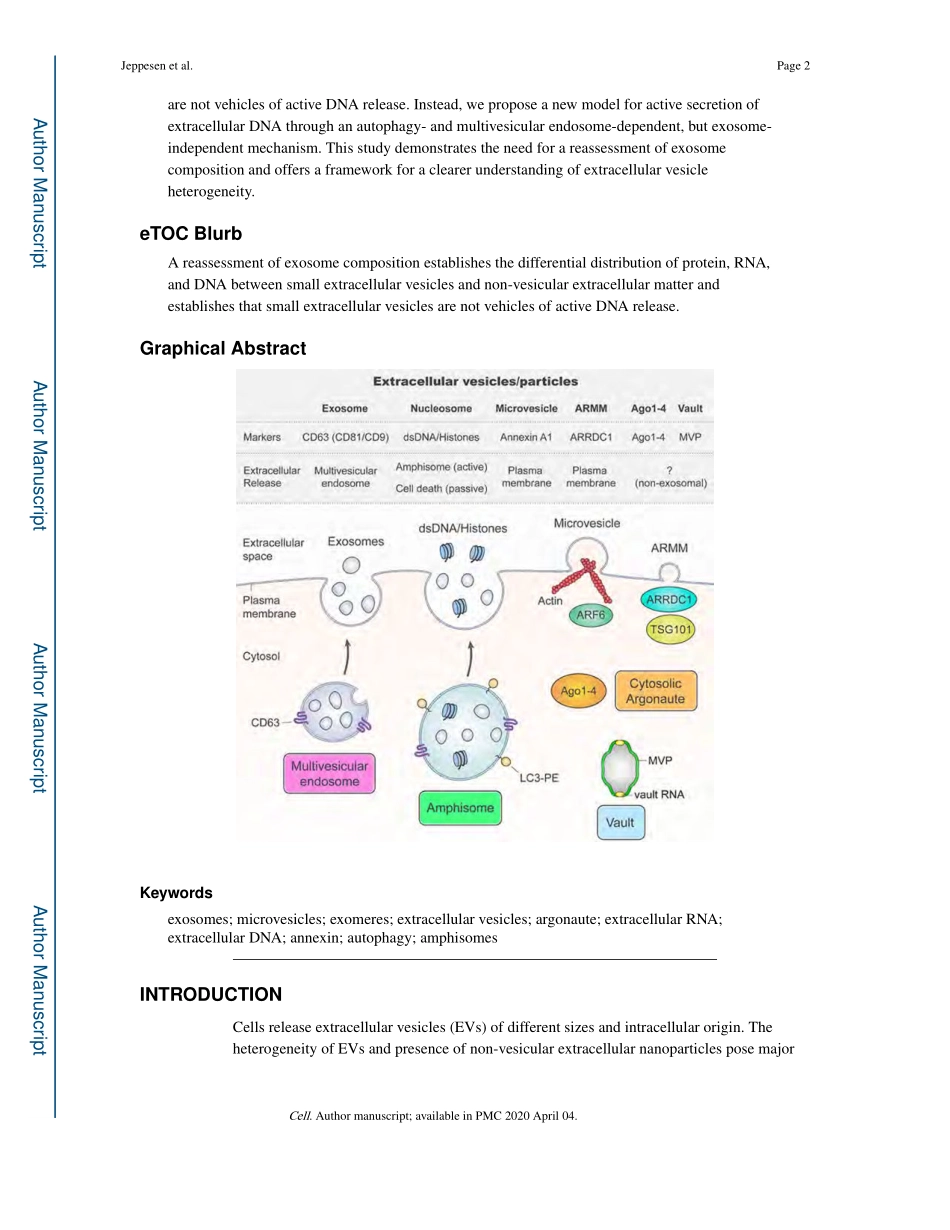
ReassessmentofExosomeCompositionDennisK.Jeppesen1,AidanM.Fenix2,JeffreyL.Franklin1,JamesN.Higginbotham1,QinZhang1,LisaJ.Zimmerman3,DanielC.Liebler3,JiePing4,QiLiu4,RachelEvans5,WilliamH.Fissell5,JamesG.Patton6,LeonardH.Rome7,DylanT.Burnette2,RobertJ.Coffey1,8,*1DepartmentofMedicine,VanderbiltUniversityMedicalCenter,Nashville,TN37232,USA2DepartmentofCellandDevelopmentalBiology,VanderbiltUniversitySchoolofMedicine,Nashville,TN37232,USA3JimAyersInstituteforPrecancerDetectionandDiagnosis,VanderbiltUniversityMedicalCenter,Nashville,TN37232,USA4DepartmentofBiostatistics,VanderbiltUniversityMedicalCenter,Nashville,TN37232,USA5DivisionofNephrologyandHypertension,VanderbiltUniversityMedicalCenter,Nashville,TN37232,USA6DepartmentofBiologicalSciences,VanderbiltUniversity,Nashville,TN37235,USA7DepartmentofBiologicalChemistry,DavidGeffenSchoolofMedicineatUniversityatCaliforniaLosAngeles,LosAngeles,CA90095,USA8LeadContact:RobertJ.Coffey,MD,EpithelialBiologyCenter,10415MRBIV,VanderbiltUniversityMedicalCenter,2213GarlandAve.,Nashville,TN37232SUMMARYTheheterogeneityofsmallextracellularvesiclesandpresenceofnon-vesicularextracellularmatterhaveledtodebateaboutcontentsandfunctionalpropertiesofexosomes.Here,weemployhigh-resolutiondensitygradientfractionationanddirectimmunoaffinitycapturetopreciselycharacterizetheRNA,DNA,andproteinconstituentsofexosomesandothernon-vesiclematerial.ExtracellularRNA,RNA-bindingproteinsandothercellularproteinsaredifferentiallyexpressedinexosomesandnon-vesiclecompartments.Argonaute1–4,glycolyticenzymesandcytoskeletalproteinsareabsentfromexosomes.WeidentifyAnnexinA1asaspecificmarkerformicrovesiclesthataresheddirectlyfromtheplasmamembrane.Wefurthershowthatsmallextracellularvesicles*Correspondence:Tel.:615-343-6228;Fax:(615)343-1591,robert.coffey@vumc.org(R.J.C.).AUTHORCONTRIBUTIONSD.K.J.conceivedthestudy,designedtheexperimentalmethodology,performedexperiments,analyzed,interpretedandvisualizedthedata,andwrotethemanuscript.A.M.F.performedexperiments,analyzedan...