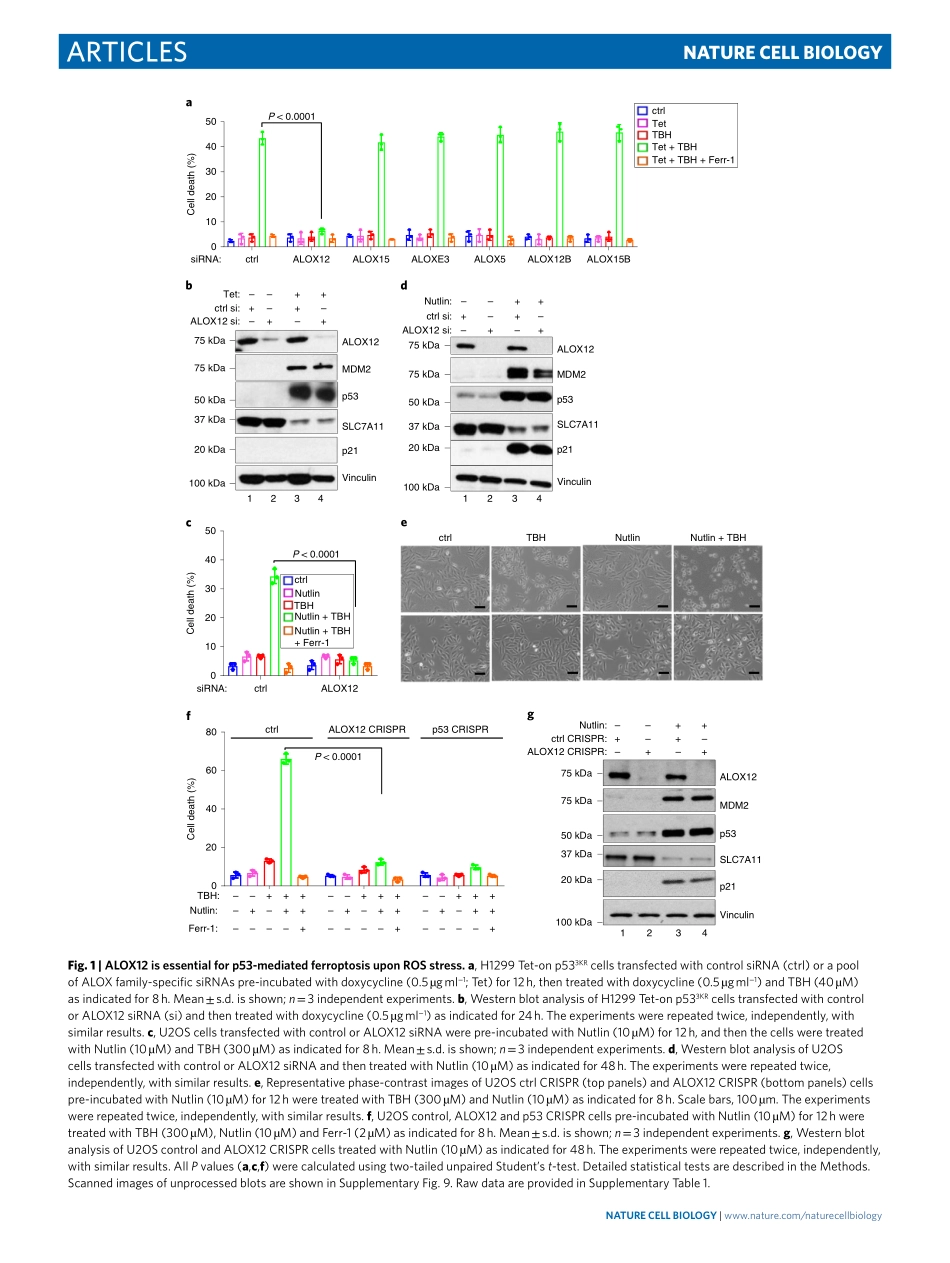
Articleshttps://doi.org/10.1038/s41556-019-0305-6InstituteforCancerGenetics,andDepartmentofPathologyandCellBiology,andHerbertIrvingComprehensiveCancerCenter,CollegeofPhysiciansandSurgeons,ColumbiaUniversity,NewYork,NY,USA.*e-mail:wg8@cumc.columbia.eduAlthoughp53-mediatedcell-cyclearrest,senescenceandapoptosisserveascriticalbarrierstocancerdevelopment,accumulatingevidencerevealsthatp53-mediatedmetabolicregulationalsopromotestumoursuppression1.Weandothershaverecentlyfoundthatp53playsanimportantroleinmodulatingfer-roptoticresponsesthroughitsmetabolictargets2–6.Nevertheless,themolecularfactorsthatmediatep53-dependentferroptosishavenotbeendelineatedandthemechanismbywhichthisferroptosispathwayisregulatedremainsunclear.Ferroptosisisaregulatedformofcelldeathdrivenbyexcessaccumulationoflipidperox-ides7–10.Lipidperoxidesarenormallyeliminatedbyglutathioneperoxidase4(GPX4)anditsco-factorglutathione(GSH),whichconvertlipidhydroperoxidestonon-toxiclipidalcohols8–10.ItiswellestablishedthatferroptosisisprimarilycontrolledbyGPX4(refs.11–19).Althoughinactivationofp53expressioncanpartiallyreduceerastin-initiatedcelldeathincertaincelltypes2–6,theferrop-toticresponsesinducedbyerastinorGPX4inhibitorsarenotdepen-dentonp53status4.Thus,itremainsunclearwhetherp53-dependentferroptosisactsthroughmodulationofGPX4function.ResultsIdentificationofALOX12asanessentialfactorofp53-dependentferroptosis.Inourpreviousstudy,weestablishedaferroptosisassaythatrequiresbothp53activationandreactiveoxygenspecies(ROS)-inducedstress(seeMethods)3.Indeed,thecelldeathinducedbylowlevelsoftert-Butylhydroperoxide(TBH),acommonROSgenera-tor,isapparentlyp53dependentandcanbespecificallyinhibitedbytheferroptosisinhibitorferrostatin-1(Ferr-1),butnotbytheinhibi-torsofothercelldeathpathwayssuchasapoptosis,autophagyornecroptosis3.Asferroptoticcelldeathistightlyregulatedinresponsetooxidativestressinvivo8,wereasonedthatthistypeofferroptosiswouldbetterreflectp53functionduringanoxidativestressresponse.InadditiontoGPX4-mediat...