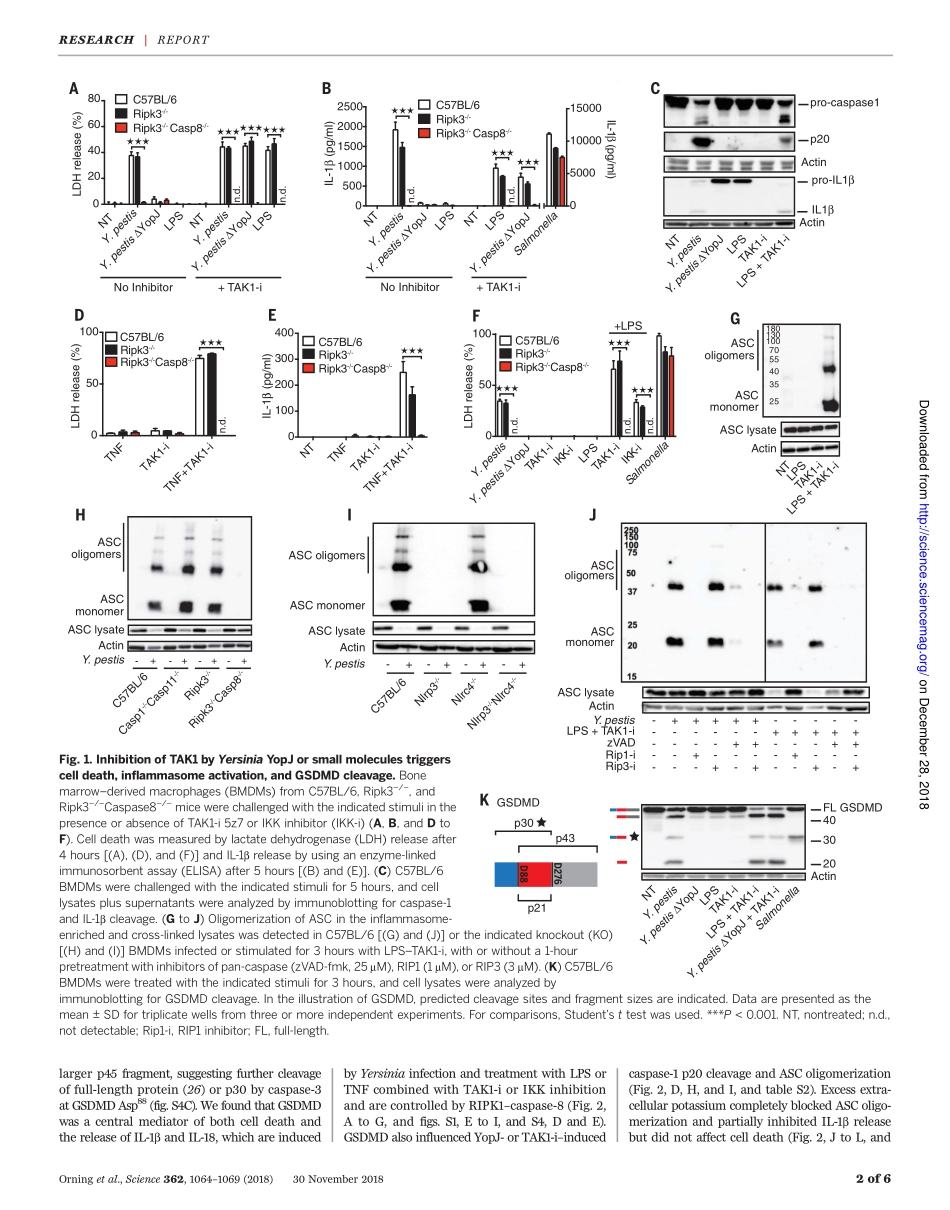
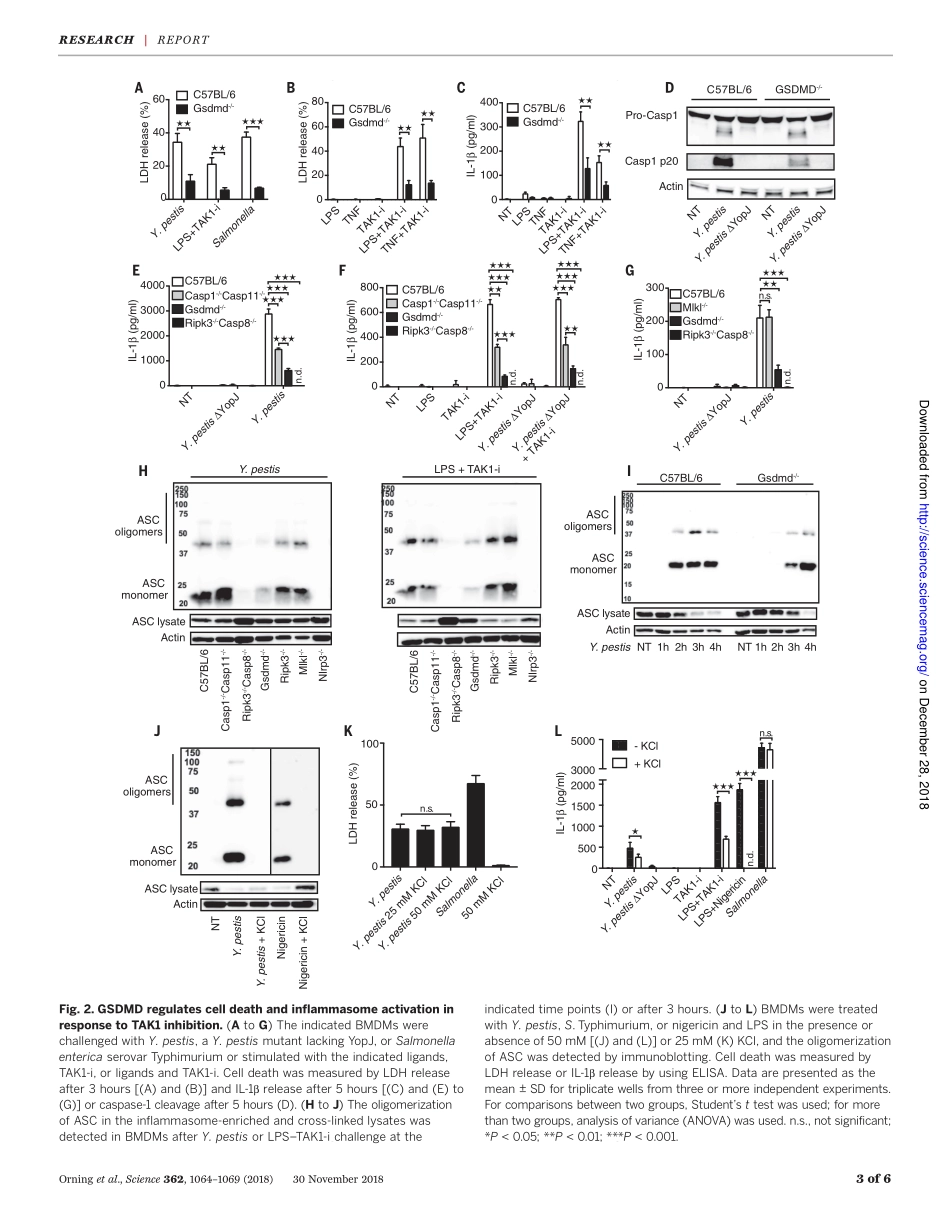
IMMUNOLOGYPathogenblockadeofTAK1triggerscaspase-8–dependentcleavageofgasderminDandcelldeathPontusOrning1,2,DanWeng1,3*,KristianStarheim1,2*,DmitryRatner1*,ZacharyBest1,BettinaLee4,AlexandriaBrooks1,ShiyuXia5,HaoWu5,MichelleA.Kelliher6,ScottB.Berger7,PeterJ.Gough7,JohnBertin7,MeganM.Proulx8,JonD.Goguen8,NobuhikoKayagaki4,KatherineA.Fitzgerald1,2,EgilLien1,2†LimitedproteolysisofgasderminD(GSDMD)generatesanN-terminalpore-formingfragmentthatcontrolspyroptosisinmacrophages.GSDMDisprocessedviainflammasome-activatedcaspase-1or-11.ItiscurrentlyunknownwhethermacrophageGSDMDcanbeprocessedbyothermechanisms.Here,wedescribeanadditionalpathwaycontrollingGSDMDprocessing.TheinhibitionofTAK1orIkBkinase(IKK)bytheYersiniaeffectorproteinYopJelicitsRIPK1-andcaspase-8–dependentcleavageofGSDMD,whichsubsequentlyresultsincelldeath.GSDMDprocessingalsocontributestotheNLRP3inflammasome–dependentreleaseofinterleukin-1b(IL-1b).Thus,caspase-8actsasaregulatorofGSDMD-drivencelldeath.Furthermore,thisstudyestablishestheimportanceofTAK1andIKKactivityinthecontrolofGSDMDcleavageandcytotoxicity.Therobustandrapidinductionofinnateimmunesignalingisahallmarkofthehostresponsetomicrobialinfection.Suc-cessfulpathogenssubvert,thwart,ordis-mantlethesedefensivemeasures.Growingevidencesuggeststhatthehostrecognizesthesedisruptiveefforts,elicitingeffectivebackupmeasures.Celldeathprocesses,includingapop-tosisandpyroptosis,areintegralcomponentsofthehostresponsetoinfection.Multiproteininflammasomecomplexessensethepresenceofpathogensandactivateinflammatorycas-pases,typicallycaspase-1orcaspase-11,leadingtopyroptoticcelldeathandmaturationoftheinflammatorycytokinesinterleukin-1b(IL-1b)andIL-18.Pyroptosisisaninflammasome-drivencytotoxicprocessthatoccursinmacrophagesafterlimitedproteolysisofgasderminD(GSDMD).ThegenerationofanN-terminallycleavedfrag-mentthencreateslargeoligomericmembraneporesandcauseslyticcelldeath(1–7).Atpresent,caspase-1andcaspase-11aretheonlyknownregulatorsofGSDMDinmacrophages(5,7),al-thoughneutroph...