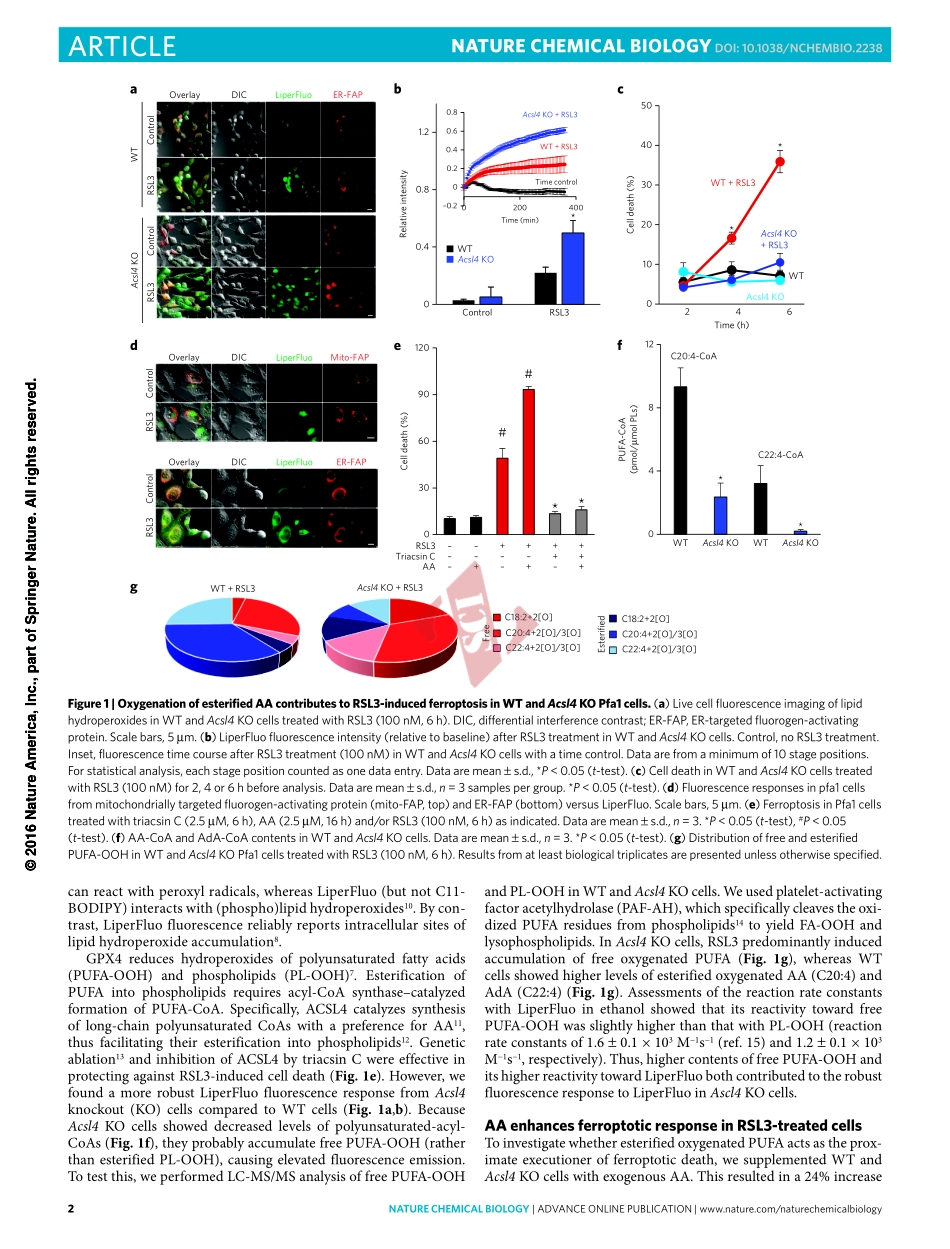
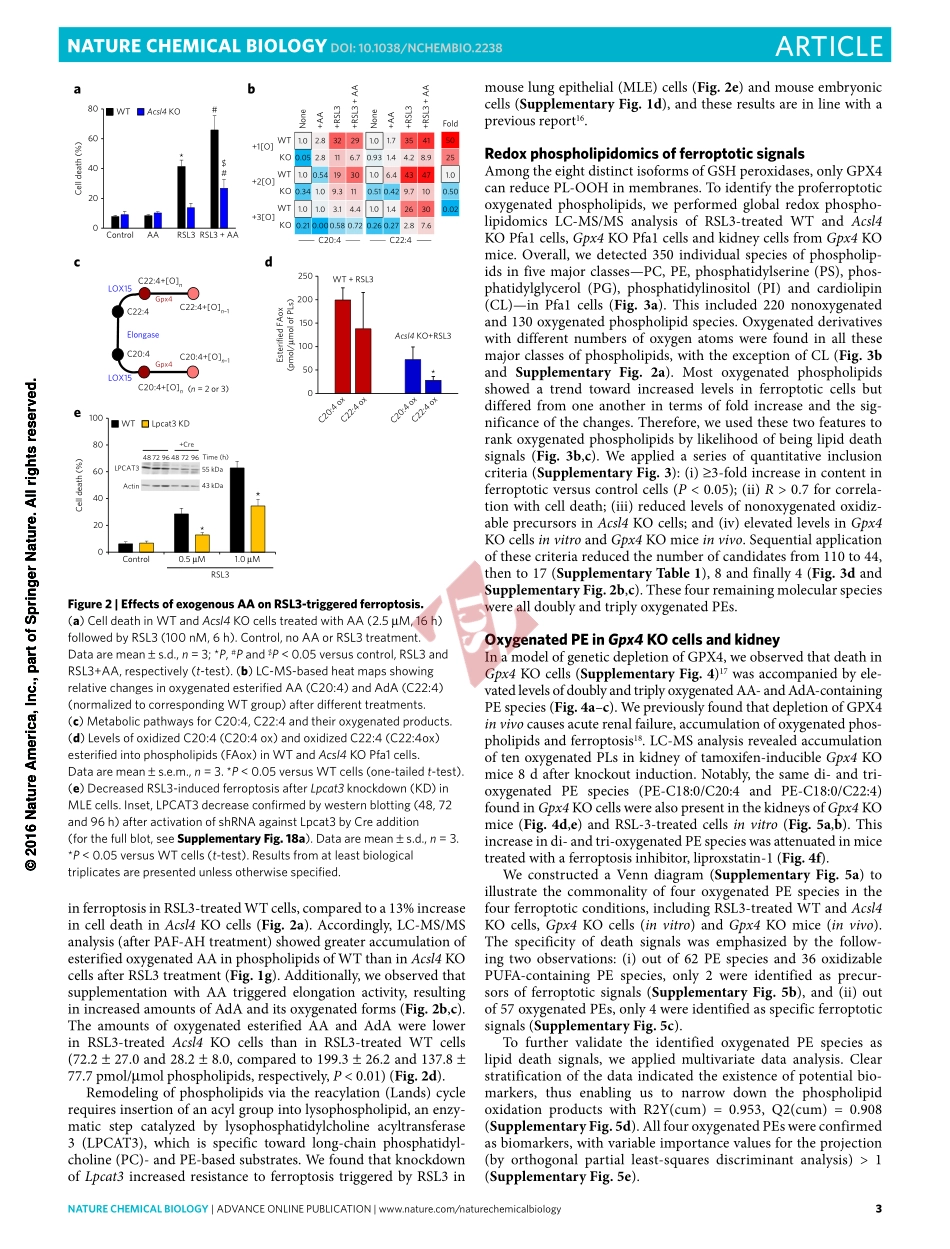
©2016NatureAmerica,Inc.,partofSpringerNature.Allrightsreserved.natureCHeMICaLBIOLOGY|AdvAnceonlinepublicAtion|www.nature.com/naturechemicalbiology1articlepuBLIsHedOnLIne:14nOveMBer2016|dOI:10.1038/nCHeMBIO.2238Multicellulareukaryoteshavedeathprogramstooptimizetis-suehomeostasis,immuneandstressresponsesandembryo-genesis1.Suchprogramsarealsobeneficialforunicellulareukaryotesand—throughquorumsensing—forbacteria,thusmakingcelldeathparadoxicallyessentialforallkingdomsoflife.Onaglobalscale,redoxferro–ferriccyclingofironbymicroorganismscontrolsthefateofthiselementintheenvironment2.Ineukaryoticcells,theelectrondonor–acceptorpropensitiesthatdefinethevitalroleofironinnormalphysiologyfacilitatetheferroptoticdeathprogram3,4.Ferroptosisisswitchedonbythedysregulationofoneofthetwomajorredoxsystems—thiolorlipidperoxidation,wherebyacombinationofGPX4orglutathione(GSH)deficiencyandacti-vationofoneormoreputativeiron-containingenzymesgener-atesoxygenatedlipidsastheproximalsignalsofdeath5.However,neitherdirectevidenceforlipidperoxidationnorthenatureoftheoxygenatedlipidspeciesresponsiblefortheferroptoticcelldemisehasbeenestablished.Here,weestablishedthatLOX,amongotheriron-containingsourcesofoxidation,candirectlyoxidizeAA-andAdA-PEintoferroptoticsignals,andthatthisprocessisfacilitatedbyACSL4-drivenesterificationofAAandAdAintoPE.RESULTSLipidhydroperoxidesaccumulateinferroptoticERToinduceGPX4deficiencyinmouseembryonicfibroblasts(Pfa1),weusedRSL3,apotentandselectiveGPX4inhibitor6.RSL3triggeredferroptoticdeath(SupplementaryResults,SupplementaryFig.1a)andcausedamarkeddecreaseinactivityofGPX4(SupplementaryFig.1b).AfterchemicalinactivationbyRSL3,weobservedamarkeddecreaseinabundanceofGPX4(SupplementaryFig.1c),suggestingthatGPX4activityisneededtopreventitsinstabilityordegradation.GPX4catalyzesthereductionofphospholipidhydroperoxidesandneutrallipidhydroperoxidestotheirrespectivehydroxyderiva-tives7.Weendeavoredtodetecttheformationoflipidhydroperox-idesinGPX4-deficientPfa1cells.Weemployedliv...