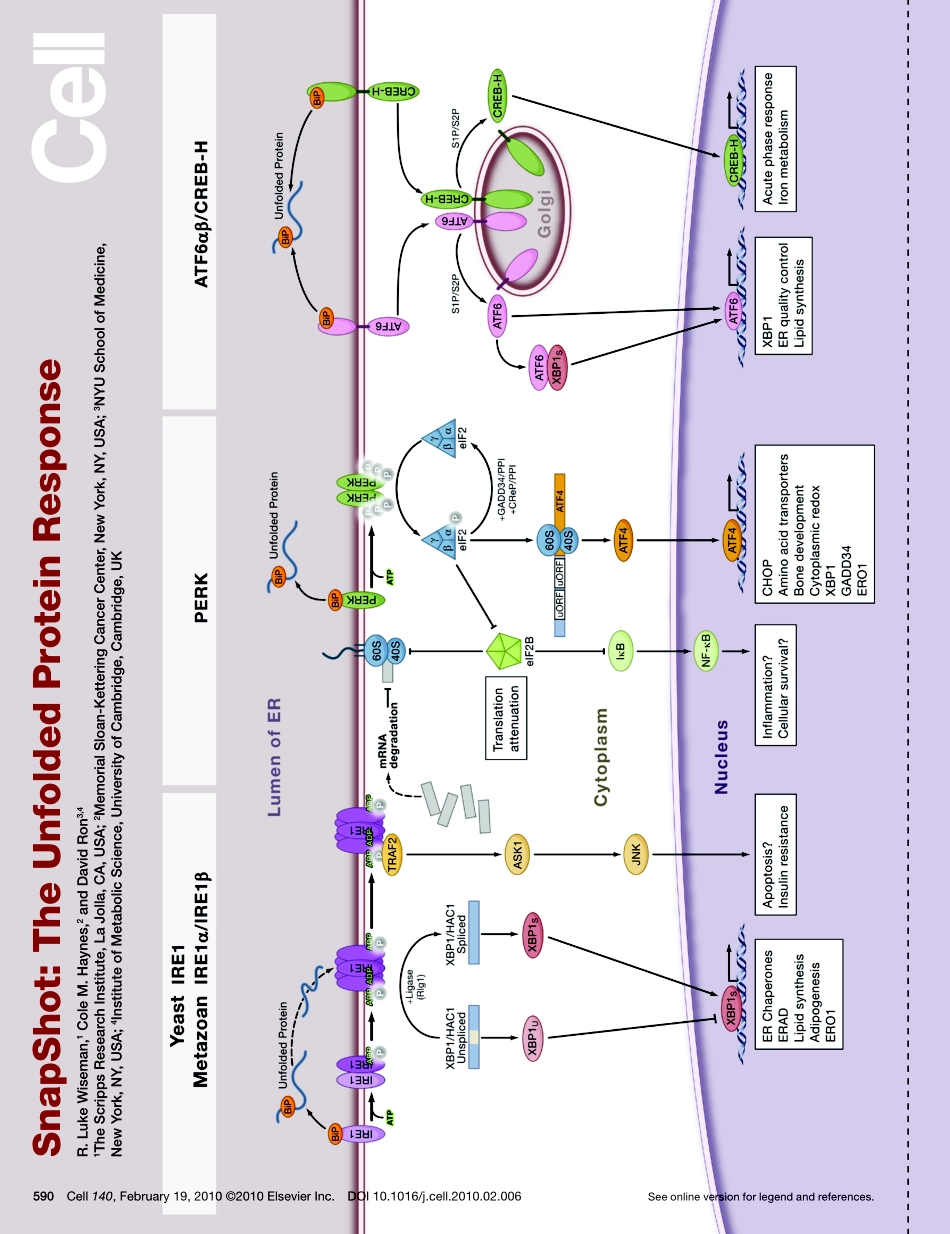
SnapShot:TheUnfoldedProteinResponseR.LukeWiseman,1ColeM.Haynes,2andDavidRon3,41TheScrippsResearchInstitute,LaJolla,CA,USA;2MemorialSloan-KetteringCancerCenter,NewYork,NY,USA;3NYUSchoolofMedicine,NewYork,NY,USA;4InstituteofMetabolicScience,UniversityofCambridge,Cambridge,UKSeeonlineversionforlegendandreferences.590Cell140,February19,2010©2010ElsevierInc.DOI10.1016/j.cell.2010.02.006SnapShot:TheUnfoldedProteinResponseR.LukeWiseman,1ColeM.Haynes,2andDavidRon3,41TheScrippsResearchInstitute,LaJolla,CA,USA;2MemorialSloan-KetteringCancerCenter,NewYork,NY,USA;3NYUSchoolofMedicine,NewYork,NY,USA;4InstituteofMetabolicScience,UniversityofCambridge,Cambridge,UK590.e1Cell140,February19,2010©2010ElsevierInc.DOI10.1016/j.cell.2010.02.006TheUnfoldedProteinResponseProteinfoldingcapacityintheendoplasmicreticulum(ER)ismatchedtothefluxofnewlysynthesizedproteinspassingthroughthesecretorypathwaybytheactivationofintra-cellularsignalingpathwayscollectivelyreferredtoastheunfoldedproteinresponse(UPR).TheUPRrespondsdirectlytotheaccumulationofunfoldedproteinsintheERlumen.AccumulationofunfoldedproteinsactivatessignalingcascadesinthecytosolviatransmembranesensorproteinsintheERmembrane.ActivationoftheUPRresultsinbothtranslationalattenuationofnewproteinsynthesisandtranscriptionalactivationofstressresponsegenestorelieveERstress.HomeostasisofproteinfoldingintheERplaysakeyroleindevelopmentandpathophysiology.Here,wepresentaSnapShotofkeyeventsintheUPRinyeastandmetazoans.UPRActivationinYeastTheUPRinthebuddingyeastSaccharomycescerevisiaeistriggeredbyasingletransmembraneproteinintheERcalledIre1.Ire1monitorsthefoldingstatusofnewlysynthe-sizedproteinsintheERlumenthroughdirectinteractionswiththeERchaperoneproteinsKar2/BiP(reviewedinRonandWalter,2007).Uponaccumulationofunfoldedproteins,Ire1isactivatedbydissociationfromKar2/BiPanddirectinteractionwiththeunfoldedproteins(dottedarrow),resultingintheoligomerizationofIre1’slumenaldomain.Signal-ingistransducedtothecytosolicdomainofIre1throughthesequentialstep...