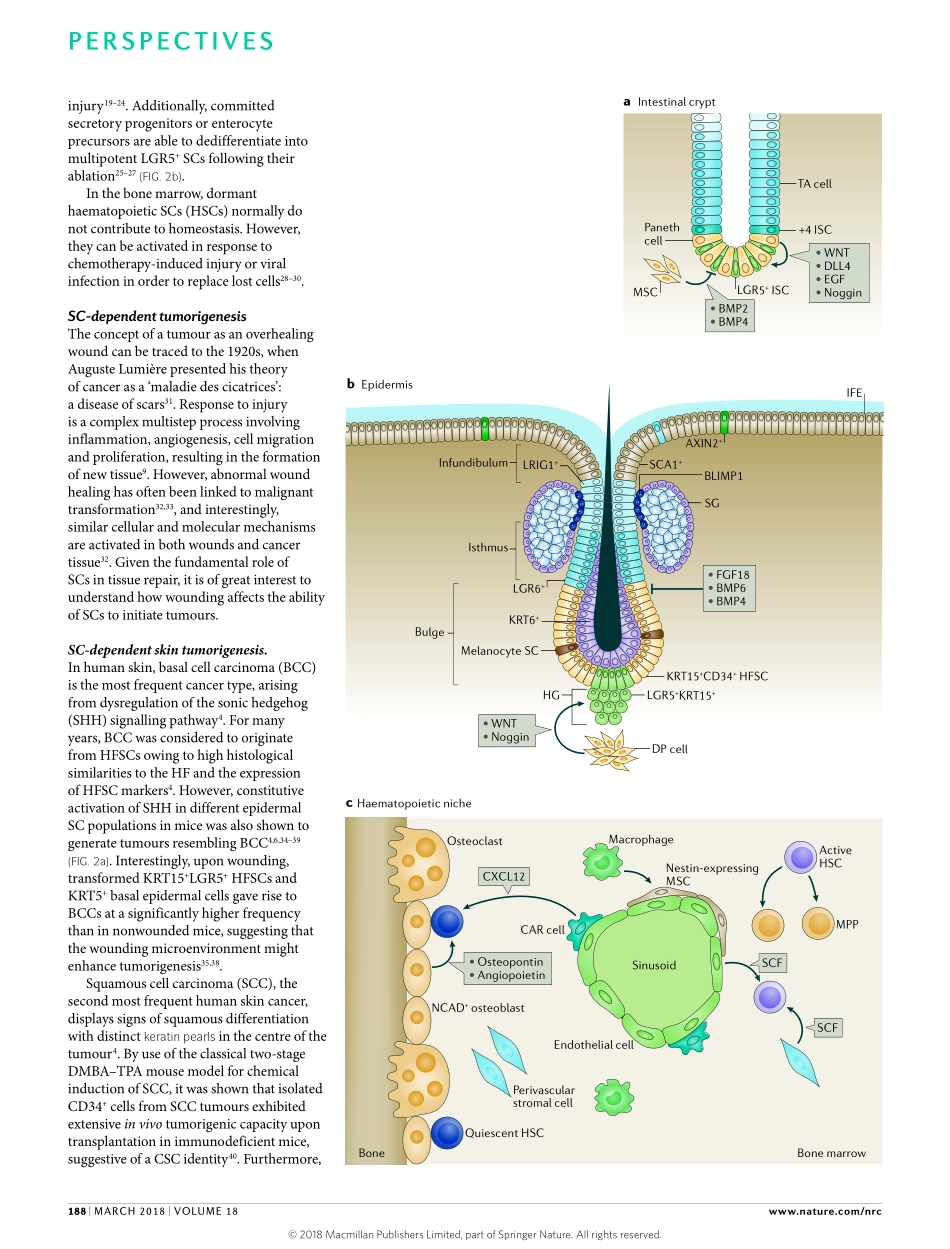
Adultstemcells(SCs)playapivotalroleintissuehomeostasis,repairandregeneration.Theseuniquecellsresideinspecializedmicroenvironments,termedstemcellniches,thataretailoredtoaccommodatetheregenerativeneedsofthetissueandregulatethefrequencyandtimingofSCself-renewal1.However,thelonglifespanofSCsincreasestheirriskofaccumulatingmutations,thusthreateningthegenomicintegrityofdaughtercells2.Inthisregard,workinrecentyearshasbeguntoshedlightonthedarkersideofSCs,arguingthatsomecancertypesdisplayahierarchicalnaturefoundedbyacancerstemcell(CSC)oramalignantcellthathasgainedSCproperties3.InthisOpinionarticle,webrieflydiscusstherolesofSCsduringthewoundhealingprocessandexplorehowcancercellscanhijackSCpropertiestopermituncontrollabletissuegrowth.WeexploreuniqueSCprotectivemechanisms,includingDNAdamageresponse(DDR)andevasionofprogrammedcelldeath(PCD).Moreover,wefocusontheeffectofsignalsemanatingfromdyingcellsandhowtheycangenerateanovelSCniche.Finally,weconsidermeansofmanipulatingSC-specificnotonlycontributetowoundhealingandhomeostasisbutalsocanserveasthecancercelloforigin4,6.SCplasticityinwoundhealingComplementingtheircriticalrolesintissuerenewal,SCsareinstrumentalinfacilitatingwoundrepairandregeneration7–9.Intheepidermis,multipleSCpopulationsofdistinctcompartmentsparticipateinre-epithelializationuponinjury.Importantly,manyofthesepopulationsdonotcontributetointerfollicularepidermis(IFE)replenishmentduringhomeostasis.ThisprovidescrucialevidencefortheplasticityofepidermalSCsandsuggeststhatlineagecommitmentcanbealteredtomeetthedemandsofthetissue10,11(FIG.2a).Forexample,followingexcisionwounds,keratin15(KRT15)+hairfollicleSCs(HFSCs),whichnormallysustainthehairfollicle(HF),temporarilycontributetoreconstructionoftheneo-epidermis,whileprogenyofleucine-richrepeat-containingGprotein-coupledreceptor6(LGR6)+SCsappeartopersistforlongerperiods12,13.AlthoughlittleisknownregardingtheSCsthatreplenishtheIFE,SCsintheIFEdivideextensivelyinresponsetowounding14.Bycontrast,existingprogenitorcells,largelyres...