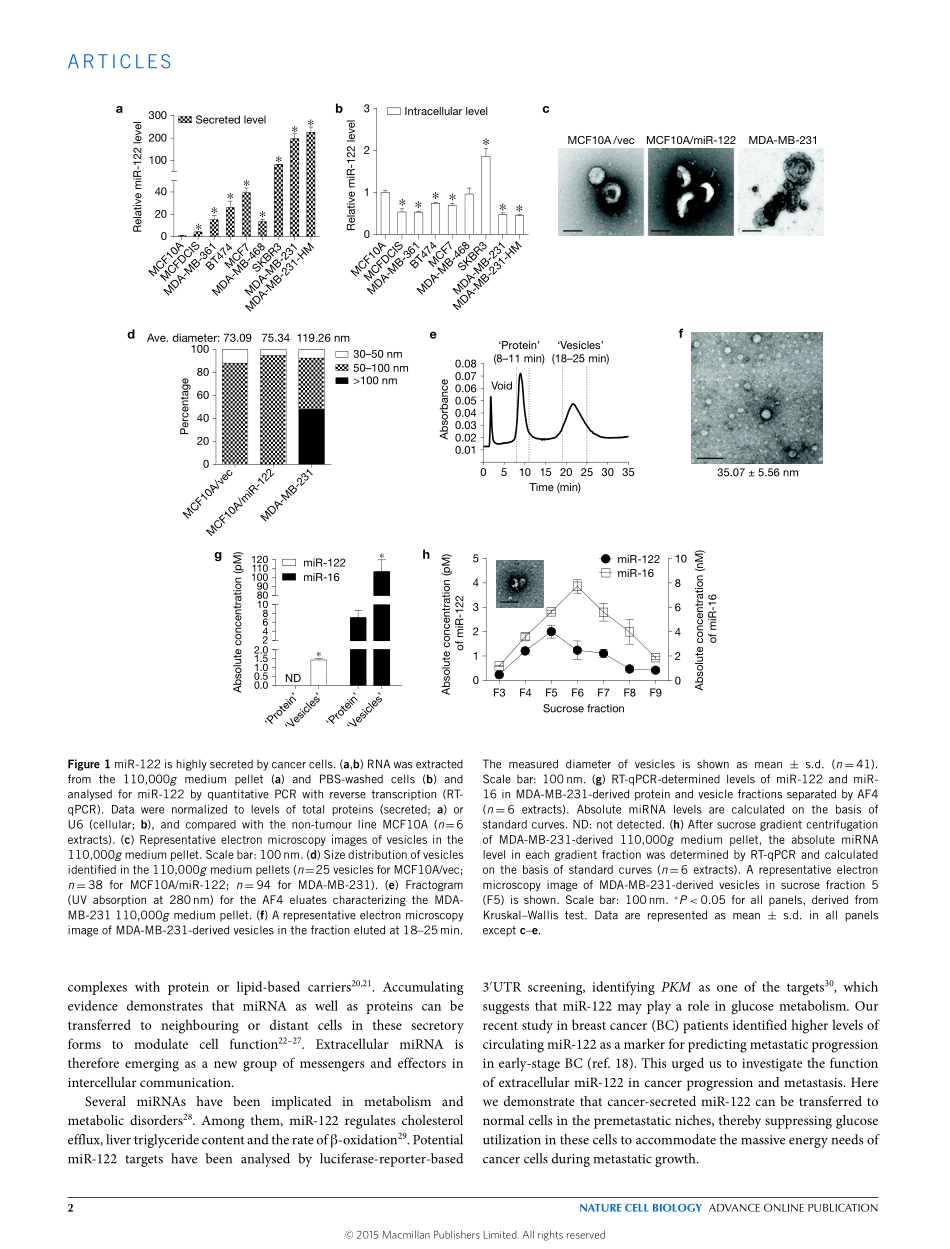
ARTICLESBreast-cancer-secretedmiR-122reprogramsglucosemetabolisminpremetastaticnichetopromotemetastasisMirandaY.Fong1,WeiyingZhou1,LiangLiu1,2,AileenY.Alontaga3,ManasaChandra1,4,JonathanAshby5,AmyChow1,SeanTimothyFrancisO’Connor1,ShashaLi1,AndrewR.Chin1,4,GeorgeSomlo6,MelaniePalomares6,7,ZhuoLi8,JacobR.Tremblay1,4,AkihiroTsuyada1,GuoqiangSun9,MichaelA.Reid1,XiweiWu10,PiotrSwiderski11,XiubaoRen2,YanhongShi9,MeiKong1,WenwanZhong5,YuanChen3andShizhenEmilyWang1,2,12Reprogrammedglucosemetabolismasaresultofincreasedglycolysisandglucoseuptakeisahallmarkofcancer.Hereweshowthatcancercellscansuppressglucoseuptakebynon-tumourcellsinthepremetastaticniche,bysecretingvesiclesthatcarryhighlevelsofthemiR-122microRNA.HighmiR-122levelsinthecirculationhavebeenassociatedwithmetastasisinbreastcancerpatients,andweshowthatcancer-cell-secretedmiR-122facilitatesmetastasisbyincreasingnutrientavailabilityinthepremetastaticniche.Mechanistically,cancer-cell-derivedmiR-122suppressesglucoseuptakebynichecellsinvitroandinvivobydownregulatingtheglycolyticenzymepyruvatekinase.InvivoinhibitionofmiR-122restoresglucoseuptakeindistantorgans,includingbrainandlungs,anddecreasestheincidenceofmetastasis.Theseresultsdemonstratethat,bymodifyingglucoseutilizationbyrecipientpremetastaticnichecells,cancer-derivedextracellularmiR-122isabletoreprogramsystemicenergymetabolismtofacilitatediseaseprogression.Reprogrammedenergymetabolismtofuelrapidcellgrowthandproliferationisanemerginghallmarkofcancer1.Mostcancercellsuseaerobicglycolysiswithreducedmitochondrialoxidativephosphorylationforglucosemetabolismevenwhenoxygenissu�cient.Thisphenomenon,knownasthe‘Warburge�ect’,favourstheuptakeandincorporationofnutrientsneededtoproduceanewcell2.TocompensatefortheconsequentreductioninATPproduction,cancercellsoftenadoptmechanismstoincreaseglucoseuptakeandutilization.Onemechanisminvolvestheregulationofglucosetransporters,amongwhichGLUT1(alsoknownasSLC2A1)isresponsibleforbasallevelsofglucoseuptakeinallcells3.GLUT1canberegulate...