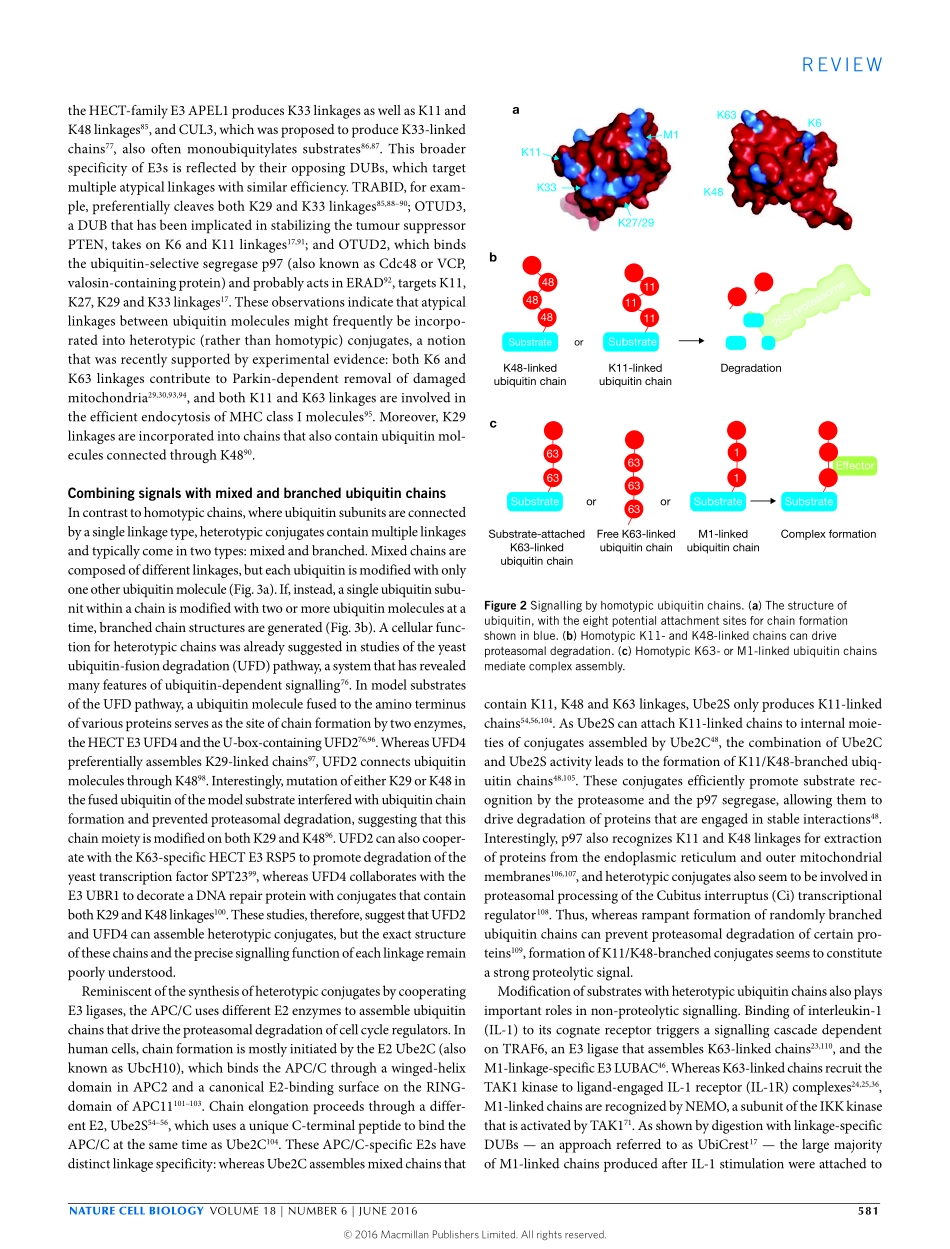
REVIEWTheincreasingcomplexityoftheubiquitincodeRichardYau1,2andMichaelRape1,2*Ubiquitylationisessentialforsignaltransductionaswellascelldivisionanddifferentiationinalleukaryotes.Substratemodificationsrangefromasingleubiquitinmoleculetocomplexpolymericchains,withdifferenttypesofubiquitylationoftenelicitingdistinctoutcomes.Therecentidentificationofnovelchaintopologieshasimprovedourunderstandingofhowubiquitylationestablishesprecisecommunicationwithincells.Here,wediscusshowtheincreasingcomplexityofubiquitylationisemployedtoensurerobustandfaithfulsignaltransductionineukaryoticcells.Communicationallowshumansocietiestothrive.Thebasisforsuchinteractionsisourabilitytoarticulatethoughtsintowordsandsentencesthatcantriggerparticularresponsesfromotherindividuals.Similarly,precisecommunicationbetweenandwithincellsisneededtosupportthedevelopmentorhomeostasisofacomplexmetazoanorganism.Thefail-ureofcellstotranslatecuesfromtheirenvironmentintoproperaction,beitdivision,differentiationorsurvival,cancausemanydiseases—adra-maticexampleiscancerresultingfromunabatedgrowthfactorsignalling.Post-translationalmodificationwiththehighlyconserved76-residueproteinubiquitinprovidescellswithanessentialmechanismtoestablishprecisecommunication.Ubiquitylationisbroughtaboutbyacascadeofthreeenzymes:E1ubiquitinactivatingenzyme,E2ubiquitinconjugatingenzyme,andE3ubiquitinproteinligase1–4.Inthesimplestversionoftheprocess,monoubiquitylation,asingleubiquitinmoleculeiscovalentlyattachedtotheε-aminogroupofalysineresidueinatargetprotein.Thisreactioncanalsooccuronmultiplelysineresiduesofthesubstratetoyieldamodificationreferredtoasmulti-monoubiquitylation(Fig.1a).TheveryefficientmonoubiquitylationofhistoneH2A,whichismediatedbythePolycombrepressivecomplexandcanaccountforupto10%ofthetotalhistoneH2Apopulation,allowedthediscoveryofthefirstsubstrateoftheubiquitinpathway5.Addingsingleubiquitinsubunitstypicallyaltersintra-orintermolecularinteractionsthatinturnaffectlocaliza-tion,complexformationoractivityofthemodifi...