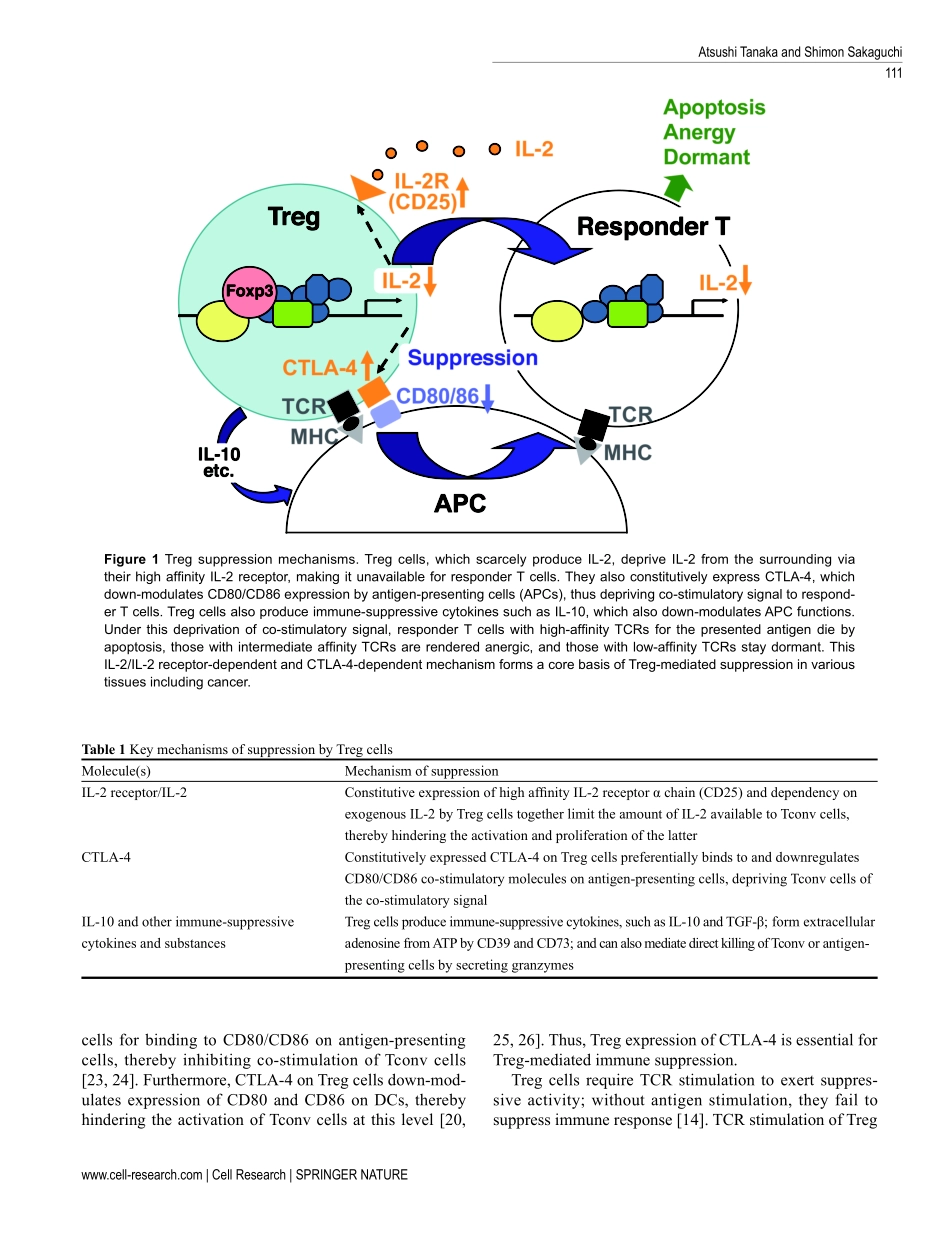
REVIEWRegulatoryTcellsincancerimmunotherapyAtsushiTanaka1,2,ShimonSakaguchi11LaboratoryofExperimentalImmunology,WPIImmunologyFrontierResearchCenter,OsakaUniversity,Suita565-0871,Japan;2DepartmentofFrontierResearchinTumorImmunology,GraduateSchoolofMedicine,OsakaUniversity,Suita565-0871,JapanFOXP3-expressingregulatoryT(Treg)cells,whichsuppressaberrantimmuneresponseagainstself-antigens,alsosuppressanti-tumorimmuneresponse.InfiltrationofalargenumberofTregcellsintotumortissuesisoftenassoci-atedwithpoorprognosis.ThereisaccumulatingevidencethattheremovalofTregcellsisabletoevokeandenhanceanti-tumorimmuneresponse.However,systemicdepletionofTregcellsmayconcurrentlyelicitdeleteriousautoim-munity.OnestrategyforevokingeffectivetumorimmunitywithoutautoimmunityistospecificallytargetterminallydifferentiatedeffectorTregcellsratherthanallFOXP3+Tcells,becauseeffectorTregcellsarethepredominantcelltypeintumortissues.Variouscellsurfacemolecules,includingchemokinereceptorssuchasCCR4,thatarespecifi-callyexpressedbyeffectorTregcellscanbethecandidatesfordepletingeffectorTregcellsbyspecificcell-depletingmonoclonalantibodies.Inaddition,otherimmunologicalcharacteristicsofeffectorTregcells,suchastheirhighex-pressionofCTLA-4,activeproliferation,andapoptosis-pronetendency,canbeexploitedtocontrolspecificallytheirfunctions.Forexample,anti-CTLA-4antibodymaykilleffectorTregcellsorattenuatetheirsuppressiveactivity.ItishopedthatcombinationofTreg-celltargeting(e.g.,byreducingTregcellsorattenuatingtheirsuppressiveactivityintumortissues)withtheactivationoftumor-specificeffectorTcells(e.g.,bycancervaccineorimmunecheckpointblockade)willmakethecurrentcancerimmunotherapymoreeffective.Keywords:Treg;CTLA-4;cancer;immunotherapyCellResearch(2017)27:109-118.doi:10.1038/cr.2016.151;publishedonline20December2016Correspondence:ShimonSakaguchiE-mail:shimon@ifrec.osaka-u.ac.jpIntroductionAnumberofstudieshaveshownthatself-antigenortumorantigen-specificCD4+andCD8+Tcellsarepresentinhealthyindividuals[1-4].Howsuchself-or...