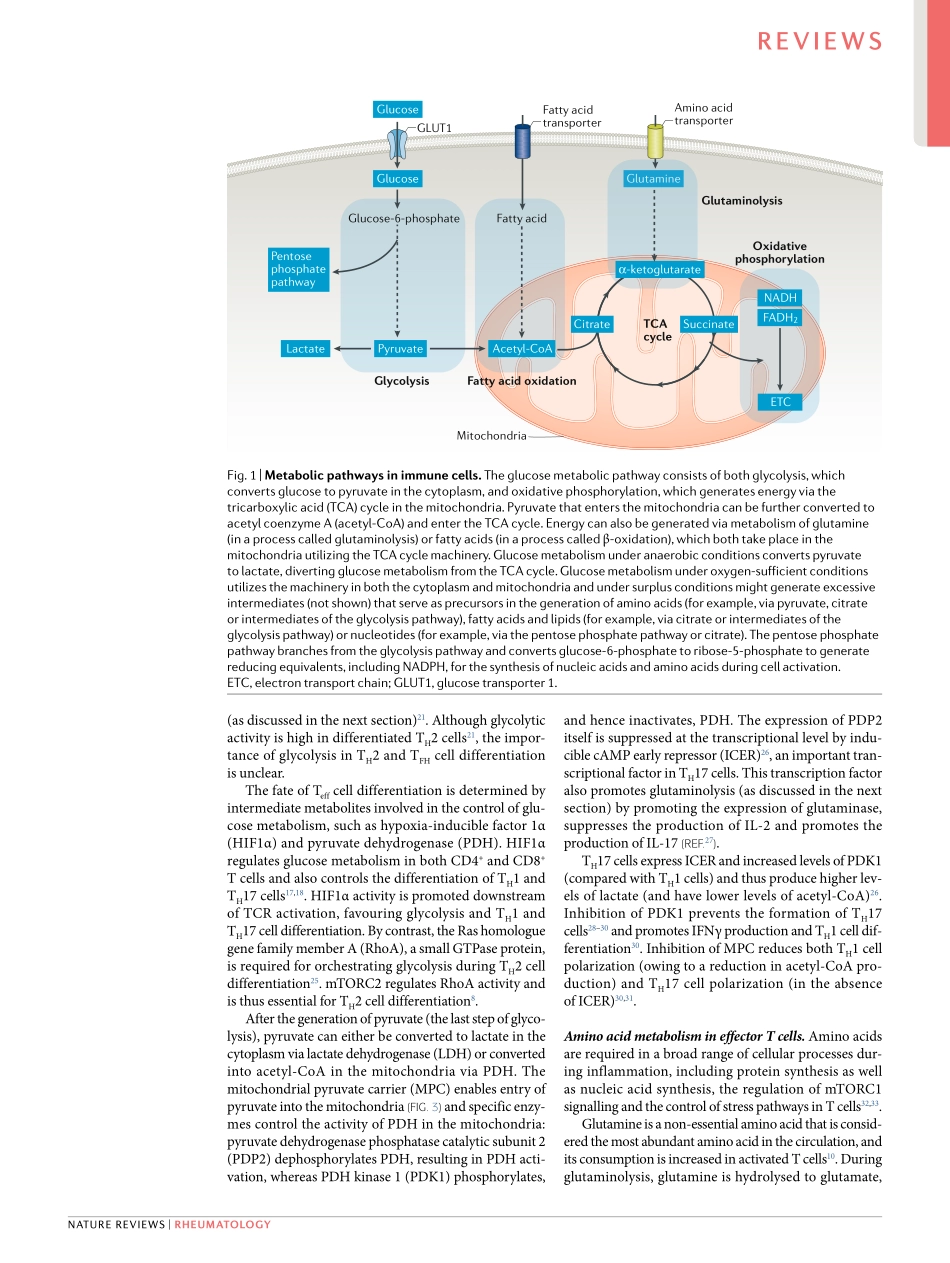
Tcellshaveanimportantfunctioninthepathogenesisofsystemiclupuserythematosus(SLE)byinstigatingandamplifyingtheinflammatoryprocessthroughdirectcontactwithotherimmunecellsinprimaryorsecondarylymphoidorgans,throughsecretingpro-inflammatorycytokinesorthroughmediatingdirecteffectsontargettissues.Manyaberrationsinthedistributionandfunc-tionofTcellsubsetshavebeendescribedinpatientswithSLEandhavebeenlinkedtotheimmunopathogenesisofSLE(asreviewedelsewhere1–3).MultiplestudieshaveidentifiedbiochemicalandmolecularabnormalitiesinTcells,includinganumberofmetabolicdisturbances4,5,thatmightexplaintheiraberrantphenotypesinpatientswithSLEandinlupus-pronemice.Pioneeringstudieshaveshowntheimportanceofalteredmetabolicpathwaysinthedevelopmentofaber-rantTcellfunctioninpatientswithSLE5andhaveshedlightonhowmoleculespreviouslylinkedtodistinctimmunecellfunctionscancontrolmetabolicenzymes6.However,ourunderstandingofthemetaboliccontrolofnormalTcellfunctionisincomplete,andtheabil-ityofmetabolicenzymestocontrolTcellfunctionunderautoimmuneconditionsislargelyunexplored.InthisReview,wefirstdescribenewdevelopmentsinourunderstandingofthemetaboliccontrolofTcellsandtheuniquerequirementsthathaveemergedforthedifferentiationofeachTcellsubset.Subsequently,wereviewthecurrentunderstandingofthecontrolofTcellfunctionbymetabolicprocessesinpatientswithSLE.Weemphasizethebiochemicalandmolecularlinksbetweenestablishedimmunemoleculesandtheexpres-sionofmetabolicenzymesandhighlightpotentialfuturetherapeutictargets.TcellmetabolismCellsrequireenergyforsurvivalandfunction,andtheprocessingofnutrientsthroughdistinctmetabolicpro-cessesproducesATPtomeettheseenergyrequirements.ThemetabolicpathwaysofTcells,aswithothercells,areaffectedbytheavailabilityofnutrientssuchasglucose,glutamineandfattyacids(Fig.1),andnutrientavailabil-itydictatesboththeactivationandfunctionofimmunecells.Glucoseisinvolvedinbothglycolysisandoxidativephosphorylation.Glycolysistakesplaceinthecytoplasmandconvertsglucosetopyruvate(generatingtwomole-culesofATP...