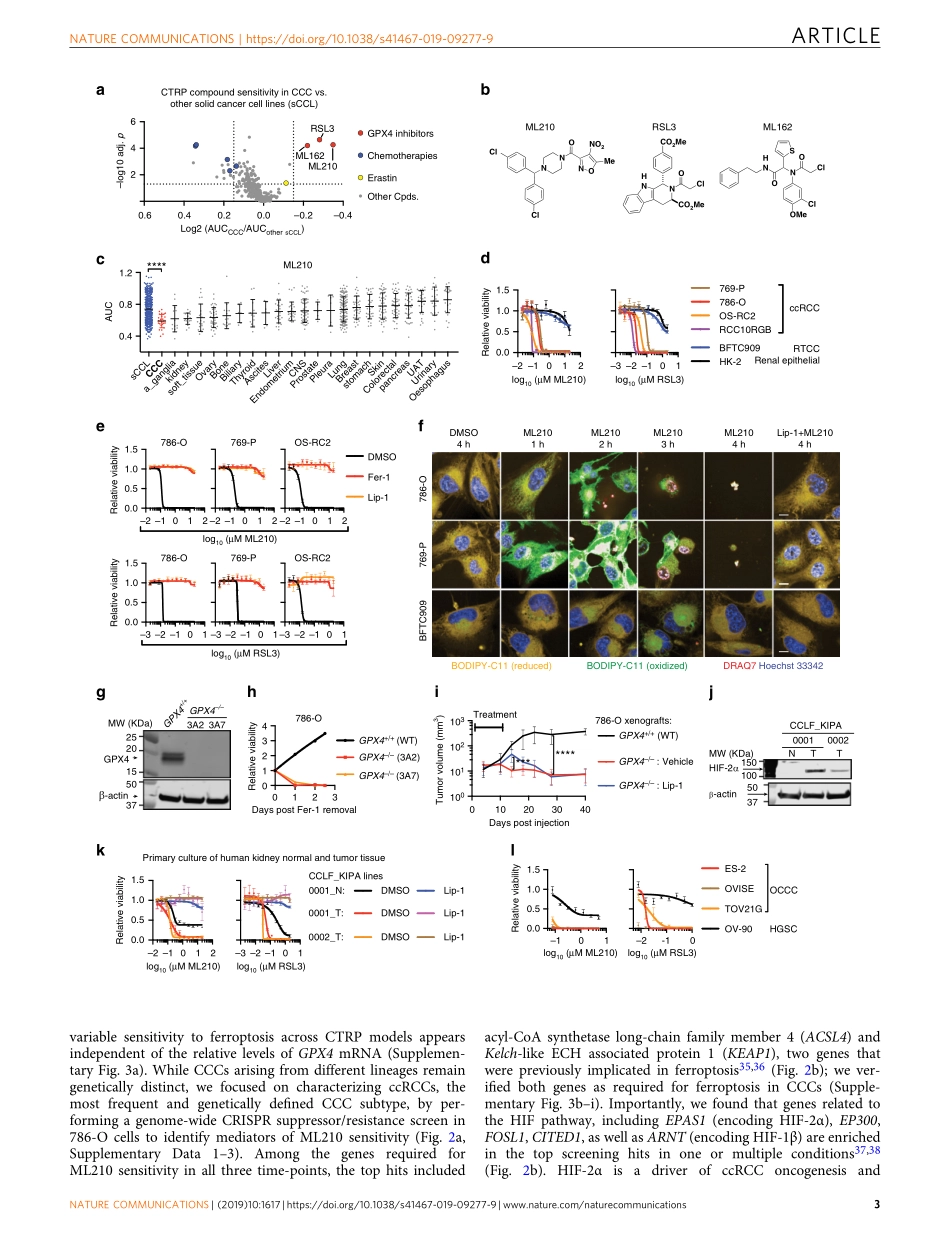
ARTICLEAGPX4-dependentcancercellstateunderliestheclear-cellmorphologyandconferssensitivitytoferroptosisYilongZou1,2,MichaelJ.Palte1,AmyA.Deik1,HaoxinLi1,2,JohnK.Eaton1,WenyuWang1,Yuen-YiTseng1,RebeccaDeasy1,MariaKost-Alimova1,VladoDančík1,ElizavetaS.Leshchiner1,VasanthiS.Viswanathan1,SabinaSignoretti3,ToniK.Choueiri4,JesseS.Boehm1,BridgetK.Wagner1,JohnG.Doench1,ClaryB.Clish1,PaulA.Clemons1&StuartL.Schreiber1,2Clear-cellcarcinomas(CCCs)areahistologicalgroupofhighlyaggressivemalignanciescommonlyoriginatinginthekidneyandovary.CCCsaredistinguishedbyaberrantlipidandglycogenaccumulationandarerefractorytoabroadrangeofanti-cancertherapies.HereweidentifyanintrinsicvulnerabilitytoferroptosisassociatedwiththeuniquemetabolicstateinCCCs.Thisvulnerabilitytranscendslineageandgeneticlandscape,andcanbeexploitedbyinhibitingglutathioneperoxidase4(GPX4)withsmall-molecules.UsingCRISPRscreeningandlipidomicprofiling,weidentifythehypoxia-induciblefactor(HIF)pathwayasadriverofthisvulnerability.InrenalCCCs,HIF-2αselectivelyenrichespolyunsaturatedlipids,therate-limitingsubstratesforlipidperoxidation,byactivatingtheexpressionofhypoxia-inducible,lipiddroplet-associatedprotein(HILPDA).OurstudysuggeststargetingGPX4asather-apeuticopportunityinCCCs,andhighlightsthattherapeuticapproachescanbeidentifiedonthebasisofcellstatesmanifestedbymorphologicalandmetabolicfeaturesinhard-to-treatcancers.https://doi.org/10.1038/s41467-019-09277-9OPEN1TheBroadInstitute,Cambridge,MA02142,USA.2DepartmentofChemistryandChemicalBiology,HarvardUniversity,Cambridge,MA02138,USA.3DepartmentofOncologicPathology,Dana-FarberCancerInstituteandBrighamandWomen’sHospital,HarvardMedicalSchool,Boston,MA02215,USA.4DepartmentofMedicalOncology,Dana-FarberCancerInstituteandBrighamandWomen’sHospital,HarvardMedicalSchool,Boston,MA02215,USA.CorrespondenceandrequestsformaterialsshouldbeaddressedtoS.L.S.(email:stuart_schreiber@harvard.edu)NATURECOMMUNICATIONS|(2019)10:1617|https://doi.org/10.1038/s41467-019-09277-9|www.nature.com/nat...