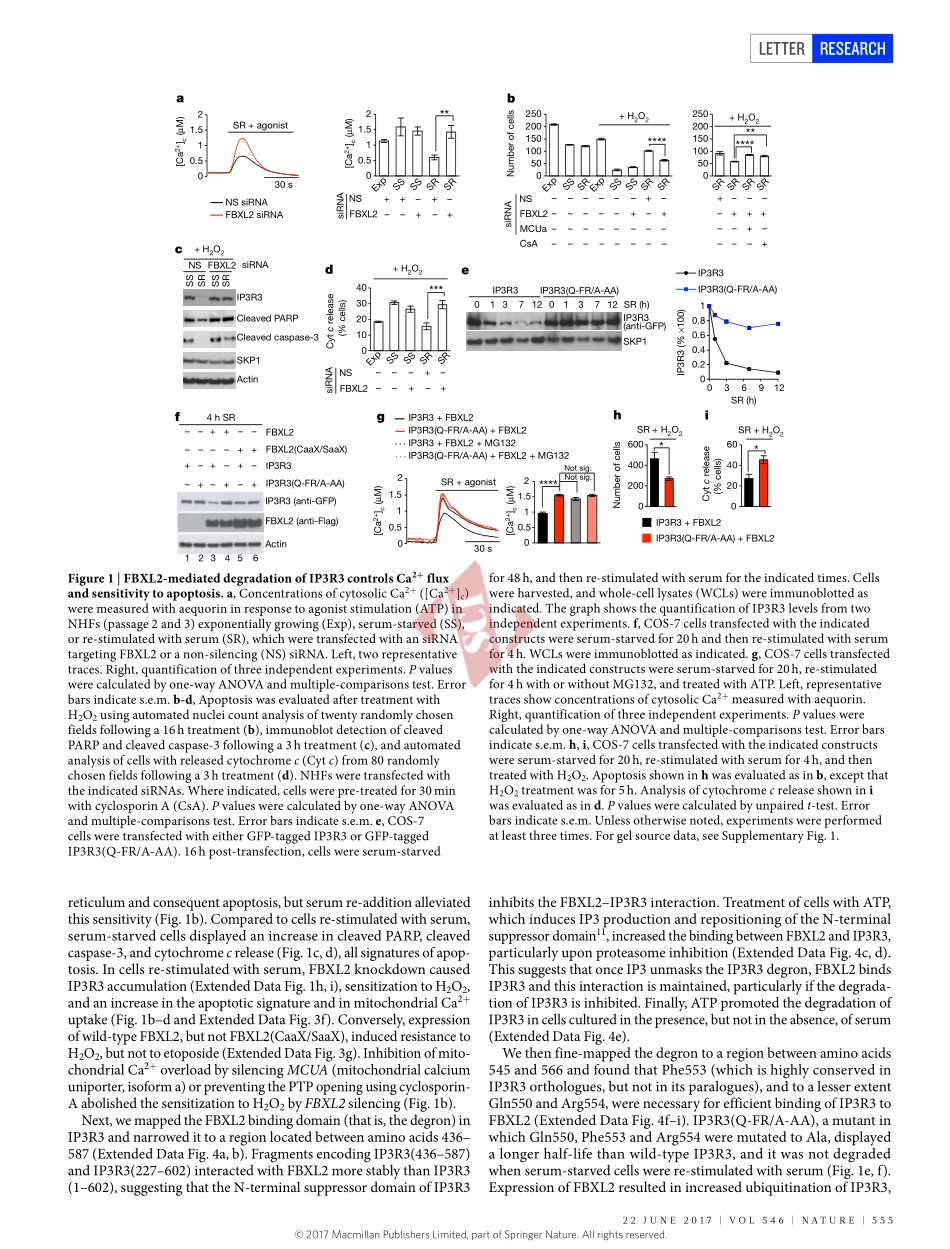
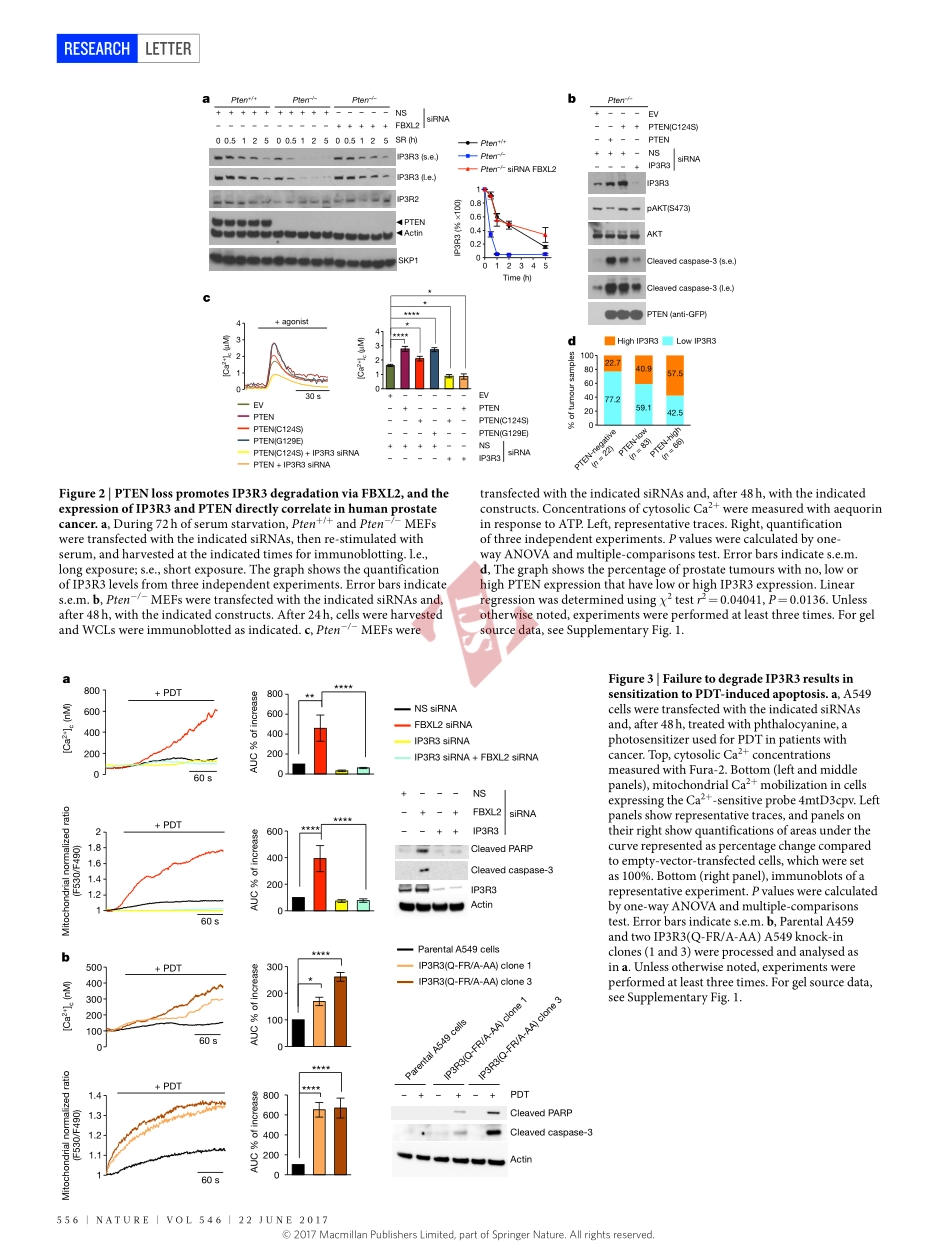
554|NATURE|VOL546|22jUNE2017LETTERdoi:10.1038/nature22965PTENcounteractsFBXL2topromoteIP3R3-andCa2+-mediatedapoptosislimitingtumourgrowthShafiKuchay1,2,3*,CarlottaGiorgi1,2,4*,DanieleSimoneschi1,2,juliaPagan1,2,3,SoniaMissiroli4,AnitaSaraf5,LaurenceFlorens5,MichaelP.Washburn5,6,AnaCollazo-Lorduy7,8,MireiaCastillo-Martin7,9,CarlosCordon-Cardo7,SaidM.Sebti10,PaoloPinton4&MichelePagano1,2,3InresponsetoenvironmentalcuesthatpromoteIP3(inositol1,4,5-trisphosphate)generation,IP3receptors(IP3Rs)locatedontheendoplasmicreticulumallowthe‘quasisynaptical’feedingofcalciumtothemitochondriatopromoteoxidativephosphorylation1.However,persistentCa2+releaseresultsinmitochondrialCa2+overloadandconsequentapoptosis2.AmongthethreemammalianIP3Rs,IP3R3appearstobethemajorplayerinCa2+-dependentapoptosis.HereweshowthattheF-boxproteinFBXL2(thereceptorsubunitofoneof69humanSCF(SKP1,CUL1,F-boxprotein)ubiquitinligasecomplexes3)bindsIP3R3andtargetsitforubiquitin-,p97-andproteasome-mediateddegradationtolimitCa2+influxintomitochondria.FBXL2-knockdowncellsandFBXL2-insensitiveIP3R3mutantknock-inclonesdisplayincreasedcytosolicCa2+releasefromtheendoplasmicreticulumandsensitizationtoCa2+-dependentapoptoticstimuli.Thephosphataseandtensinhomologue(PTEN)geneisfrequentlymutatedorlostinhumantumoursandsyndromesthatpredisposeindividualstocancer4.WefoundthatPTENcompeteswithFBXL2forIP3R3binding,andtheFBXL2-dependentdegradationofIP3R3isacceleratedinPten−/−mouseembryonicfibroblastsandPTEN-nullcancercells.ReconstitutionofPTEN-nullcellswitheitherwild-typePTENoracatalyticallydeadmutantstabilizesIP3R3andinducespersistentCa2+mobilizationandapoptosis.IP3R3andPTENproteinlevelsdirectlycorrelateinhumanprostatecancer.Bothincellcultureandxenograftmodels,anon-degradableIP3R3mutantsensitizestumourcellswithlowornoPTENexpressiontophotodynamictherapy,whichisbasedontheabilityofphotosensitizerdrugstocauseCa2+-dependentcytotoxicityafterirradiationwithvisiblelight5,6.Similarly,disruptionofFBXL2localizationwithGGTi-2418,ageranylge...