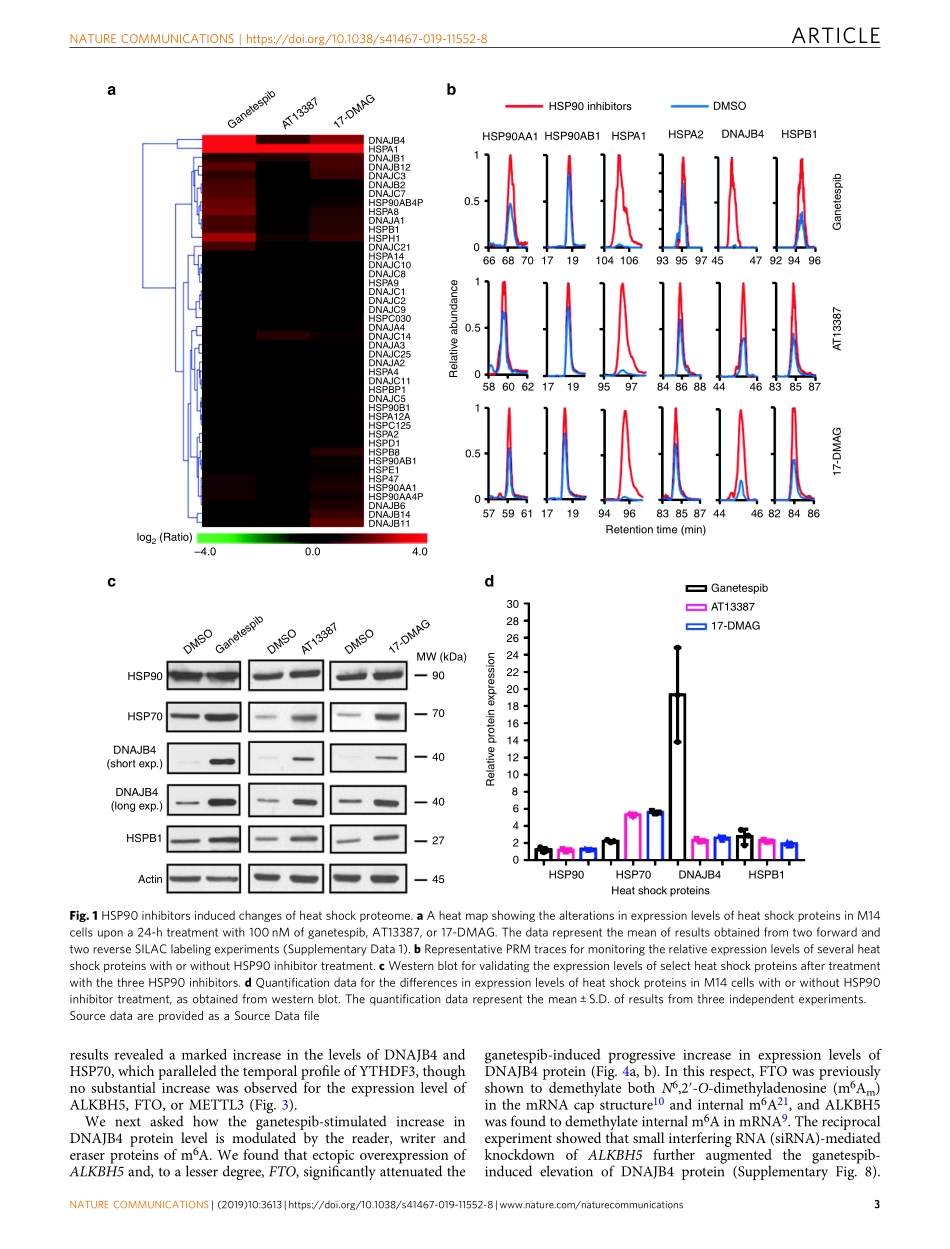
ARTICLEHSP90inhibitorsstimulateDNAJB4proteinexpressionthroughamechanisminvolvingN6-methyladenosineWeiliMiao1,LinLi1,YonghuiZhao2,3,XiaoxiaDai1,XuemeiChen2&YinshengWang1Small-moleculeinhibitorsforthe90-kDaheatshockprotein(HSP90)havebeenextensivelyexploitedinpreclinicalstudiesforthetherapeuticinterventionsofhumandiseasesaccom-paniedwithproteotoxicstress.Byusinganunbiasedquantitativeproteomicmethod,weuncoverthattreatmentwiththreeHSP90inhibitorsresultsinelevatedexpressionofalargenumberofheatshockproteins.WealsodemonstratethattheHSP90inhibitor-mediatedincreaseinexpressionofDNAJB4proteinoccurspartlythroughanepitranscriptomicmechanism,andissubstantiallymodulatedbythewriter,eraser,andreaderproteinsofN6-methyladenosine(m6A).Furthermore,exposuretoganetespibleadstoelevatedmodificationlevelsatm6Amotifsitesinthe5′-UTRofDNAJB4mRNA,andthemethylationatadenosine114siteinthe5′-UTRpromotesthetranslationofthereportergenemRNA.Thism6A-mediatedmechanismisalsoatplayuponheatshocktreatment.Cumulatively,weunveilthatHSP90inhibitorsstimulatethetranslationofDNAJB4throughanepitranscriptomicmechanism.https://doi.org/10.1038/s41467-019-11552-8OPEN1DepartmentofChemistry,UniversityofCaliforniaRiverside,Riverside,CA92521-0403,USA.2DepartmentofBotanyandPlantSciences,UniversityofCaliforniaRiverside,Riverside,CA92521-0403,USA.3GuangdongProvincialKeyLaboratoryforPlantEpigenetics,CollegeofLifeSciencesandOceanography,ShenzhenUniversity,518060Shenzhen,Guangdong,China.CorrespondenceandrequestsformaterialsshouldbeaddressedtoY.W.(email:Yinsheng.Wang@ucr.edu)NATURECOMMUNICATIONS|(2019)10:3613|https://doi.org/10.1038/s41467-019-11552-8|www.nature.com/naturecommunications11234567890():,;RNAisknowntoharbormorethan100typesofcovalentmodifications1,andthebiologicalfunctionsformostofthesemodificationsremainpoorlyunderstood.RecentstudiesdocumentedthewidespreadoccurrenceofN6-methy-ladenosine(m6A)inmRNAandthediscoveryofcellularproteinsthatareinvolvedinthedeposition2–4,recognition5–7,andremoval8–10ofthismodifiednuc...