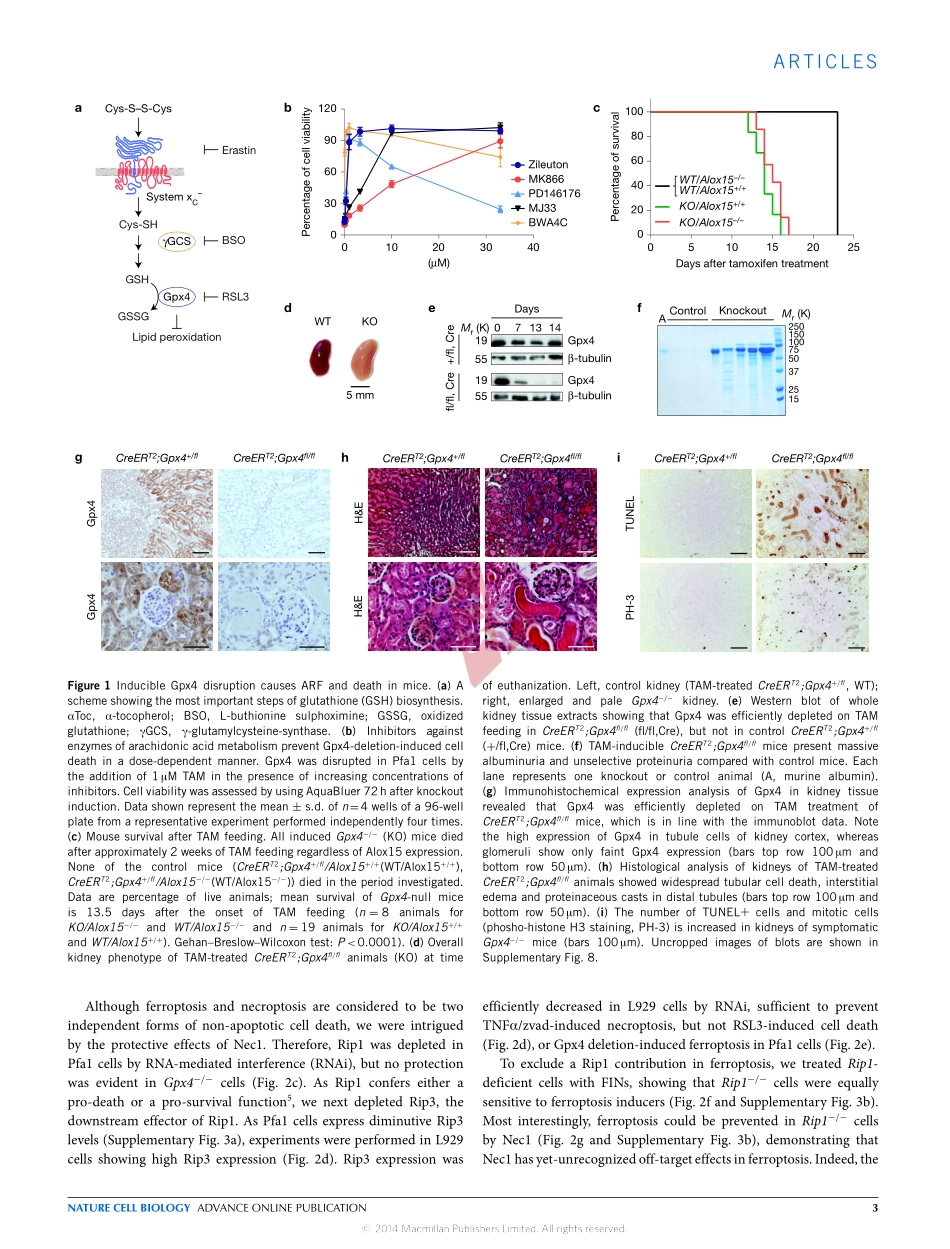
ARTICLESInactivationoftheferroptosisregulatorGpx4triggersacuterenalfailureinmiceJosePedroFriedmannAngeli1,ManuelaSchneider2,BettinaProneth1,YuliaY.Tyurina3,VladimirA.Tyurin3,VictoriaJ.Hammond4,NadjaHerbach5,MichaelaAichler6,AxelWalch6,ElkeEggenhofer7,DevarajBasavarajappa8,OlofRådmark8,ShoKobayashi1,9,TobiasSeibt1,HeikeBeck10,FraukeNeff6,IreneEsposito11,RüdigerWanke5,HeidiFörster1,OlenaYefremova1,MarcHeinrichmeyer1,GeorgW.Bornkamm12,EdwardK.Geissler7,StephenB.Thomas13,BrentR.Stockwell13,ValerieB.O’Donnell4,ValerianE.Kagan3,JoelA.Schick1andMarcusConrad1,14Ferroptosisisanon-apoptoticformofcelldeathinducedbysmallmoleculesinspecifictumourtypes,andinengineeredcellsoverexpressingoncogenicRAS.Yet,itsrelevanceinnon-transformedcellsandtissuesisunexploredandremainsenigmatic.Here,weprovidedirectgeneticevidencethattheknockoutofglutathioneperoxidase4(Gpx4)causescelldeathinapathologicallyrelevantformofferroptosis.UsinginducibleGpx4−/−mice,weelucidateanessentialrolefortheglutathione/Gpx4axisinpreventinglipid-oxidation-inducedacuterenalfailureandassociateddeath.Wefurthermoresystematicallyevaluatedalibraryofsmallmoleculesforpossibleferroptosisinhibitors,leadingtothediscoveryofapotentspiroquinoxalinaminederivativecalledLiproxstatin-1,whichisabletosuppressferroptosisincells,inGpx4−/−mice,andinapre-clinicalmodelofischaemia/reperfusion-inducedhepaticdamage.Insum,wedemonstratethatferroptosisisapervasiveanddynamicformofcelldeath,which,whenimpeded,promisessubstantialcytoprotection.Previouslyitwasassumedthatapoptosiswastheonlyregulatedformofcelldeath.Yet,recentyearshaveshownthatnon-apoptoticcelldeathpathwaysarehighlyheterogeneousprocessescharacterizedbymorphologicallyandbiochemicallydistinctevents1.Non-apoptoticcelldeathpathwayshavegainedspecialattentionparticularlyduetotheirroleininflammationandaspotentialtherapeutictargetinapoptosis-resistanttumours2,3.Inthiscontext,atleasttwocelldeathpathwaysareexecutedinresponsetodetrimentalconcentrationsofpartiallyreducedformsofoxygen(thatis,...