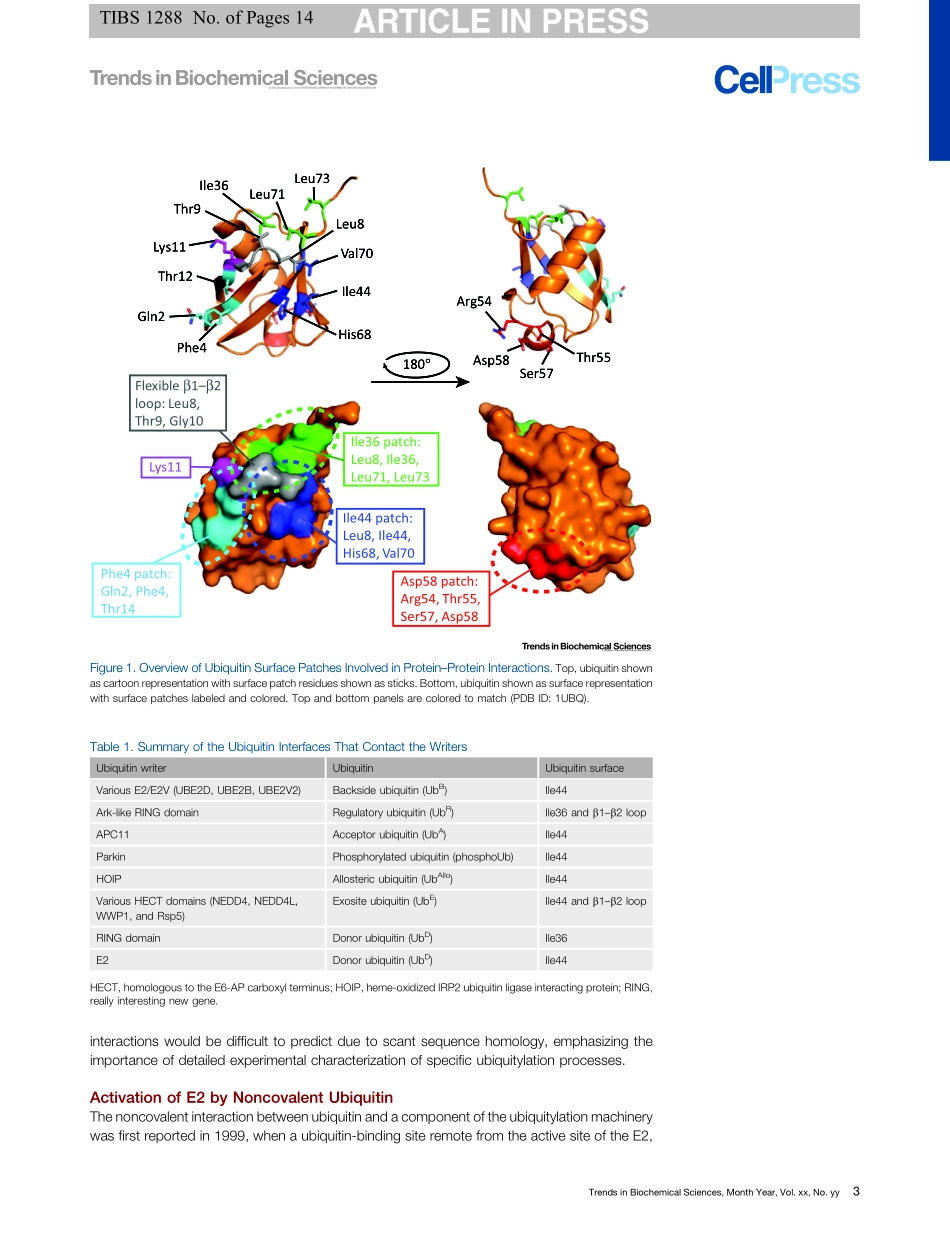
ReviewNoncovalentUbiquitinInteractionsRegulatetheCatalyticActivityofUbiquitinWritersJoshuaD.Wright,1,2PeterD.Mace,1andCatherineL.Day1,*Covalentmodificationofsubstrateproteinswithubiquitinistheendresultofanintricatenetworkofprotein–proteininteractions.TheinherentabilityoftheE1,E2,andE3proteinsoftheubiquitylationcascade(theubiquitinwriters)tointeractwithubiquitinfacilitatesthisprocess.Importantly,contactbetweenubiquitinandtheE2/E3writersisrequiredforcatalysisandtheassemblyofchainsofagivenlinkage.However,ubiquitinisalsoanactivatorofubiquitin-writingenzymes,withmanyrecentstudieshighlightingtheabilityofubiquitintoregulateactivityandsubstratemodification.Here,wereviewtheinteractionsbetweenubiquitin-writingenzymesandregulatoryubiquitinmoleculesthatpromoteactivity,andhighlightthepotentialoftheseinteractionstopromoteprocessiveubiquitintransfer.WritingtheUbiquitinCodeModificationofproteinsbytheadditionofubiquitin(itselfa76-residueprotein)regulatesvirtuallyallbiochemistrywithineukaryoticcells.Ubiquitylationrequirestheconcertedactivitiesofthreedistinctclassesofubiquitin-writingenzymes[49_TD$DIF]–[50_TD$DIF]anATP-dependentubiquitinactivatingenzyme(E1;seeGlossary),aubiquitin-conjugating(UBC)enzyme(E2,30�40inhumans),andaubiquitinligase(E3,>500inhumans)[1](Box1).ThereareseveralclassesofE3sthatemploydistinctmechanismstopromoteubiquitintransfer(Box1),butirrespectiveofthemechanism,togethertheE2sandE3srecruitthesubstrateandspecifythetypeofubiquitinmodification[2].Thefinalubiquitinmodificationattachedtosubstrateproteinsishighlyvariable,includingsingleubiquitinmoietiesorpolyubiquitinchainsofeightdistinctlinkages[3].Thesedifferenttypesofubiquitinmodificationscanbethoughtofastheubiquitincode,whichisinterpretedbyubiquitinreadersanddictatesthefateofthesubstrate.Forexample,proteinscovalentlymodifiedwithLys48-linkedpolyubiquitinchainsarerecognized/readanddegradedbytheproteasome[4],whereasLys63-linkedchainsmediatetheassemblyofproteincomplexes[5].EventhoughE2sandE3swritetheubiquitincode,an...