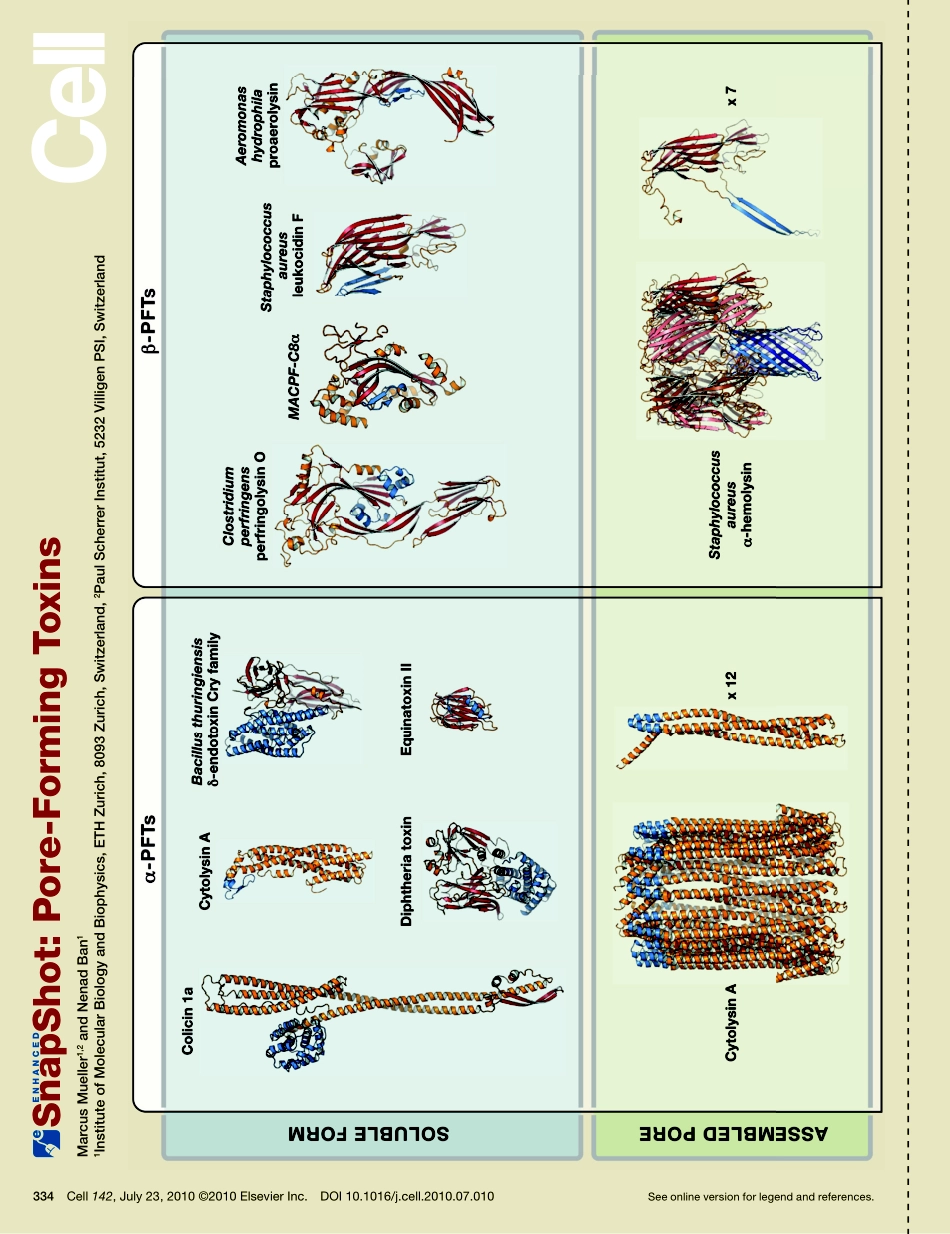
SnapShot:Pore-FormingToxinsMarcusMueller1,2andNenadBan11InstituteofMolecularBiologyandBiophysics,ETHZurich,8093Zurich,Switzerland,2PaulScherrerInstitut,5232VilligenPSI,SwitzerlandSeeonlineversionforlegendandreferences.334Cell142,July23,2010©2010ElsevierInc.DOI10.1016/j.cell.2010.07.010SnapShot:Pore-FormingToxinsMarcusMueller1,2andNenadBan11InstituteofMolecularBiologyandBiophysics,ETHZurich,8093Zurich,Switzerland,2PaulScherrerInstitut,5232VilligenPSI,Switzerland334.e1Cell142,July23,2010©2010ElsevierInc.DOI10.1016/j.cell.2010.07.010Pore-formingtoxins(PFTs)arealargeclassofvirulencefactorsthatincludesomeofnature’smostpotentbiologicalweapons.Theyareemployedbyawiderangeofbacterialpathogensbutalsooccurineukaryoteswheretheyplayrolesinimmunedefenseorasvenoms.Astheirnameimplies,PFTsformporesinthemembranesoftargetcells.ThetoxiceffectofPFTsiseitherdisruptionofthepermeabilitybarriercausingleakageorthedeliveryoftoxiccomponentsthroughthepores.OneremarkablecharacteristicofPFTsistheirtransformationfromasoluble,monomericproteintoanoligomerictransmembranechan-nelwithfeaturesofanintegralmembraneprotein(ParkerandFeil,2005).Forthistransformationthetoxinmustundergoconformationalchangesinwhichhydrophobicpatchesthatareburiedinthesolublestatebecomeexposedtothehydrophobicenvironmentofthemembranelipids.PFTscanformbothasolubleandamembraneproteinfromthesamepolypeptideandtherebychallengethenotionthatthesetypesofproteinsarenecessarilydistinct.Despitethebroaddiversityinsequenceandstructurebetweendiffer-entfamiliesofPFTstheytypicallyactbyacommonmechanism.Thetoxinissecretedinitssolubleformanddiffusestothetargetcell.BindingtothetargetcellismediatedbyspecificreceptorsthathavebeenidentifiedformanyPFTsandincludecellsurfacefeaturessuchasmembrane-orglycophosphatidylinositol-anchoredproteinsorlipids.Oligomerizationandassemblyoftheporeisthenfollowedbymembraneintegration.PFTscanbeclassifiedasα-PFTsandβ-PFTs,dependingonthetypesofsecondarystructuretheyusetoinsertintothemembrane.α-PFTsspanthetargetmembr...