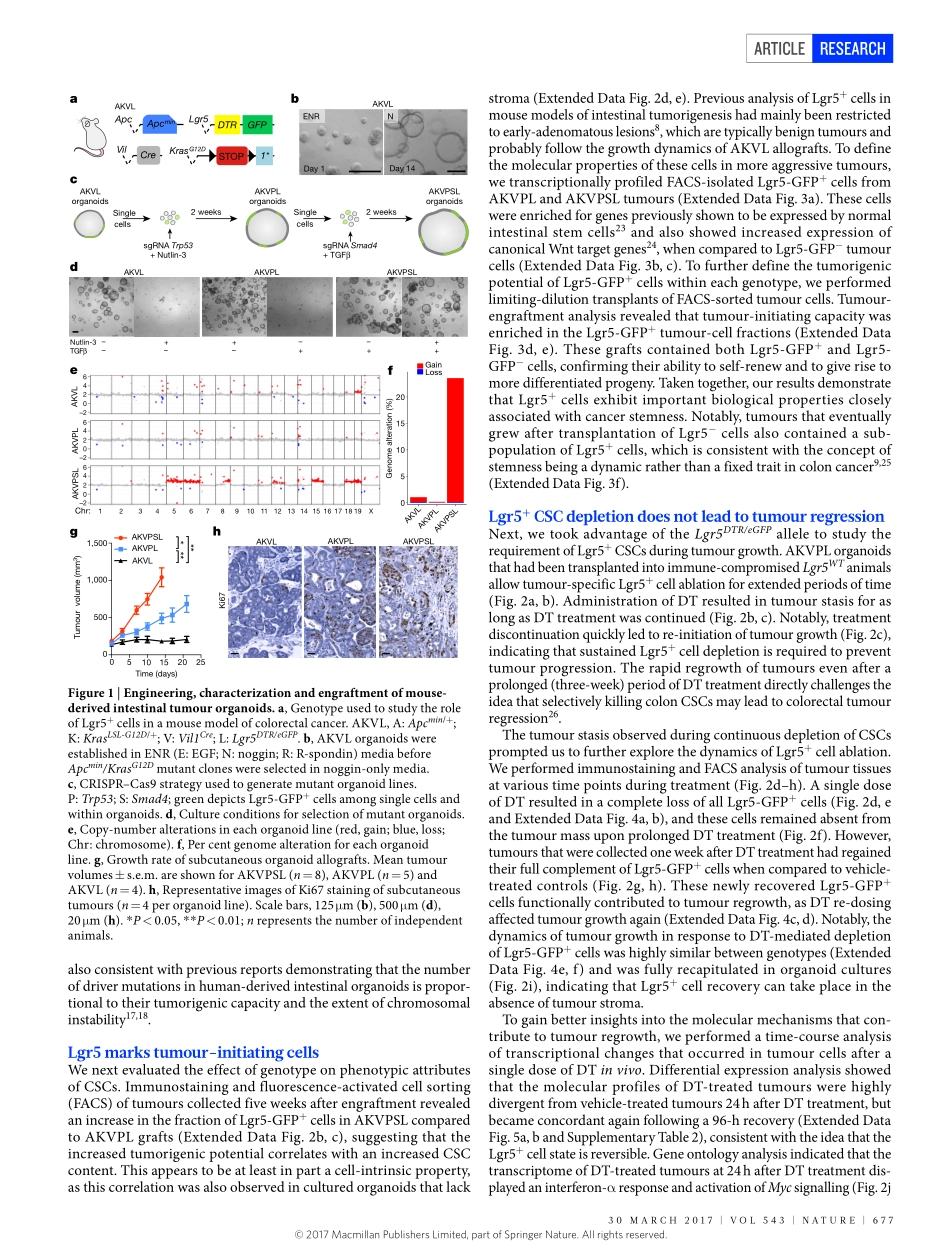
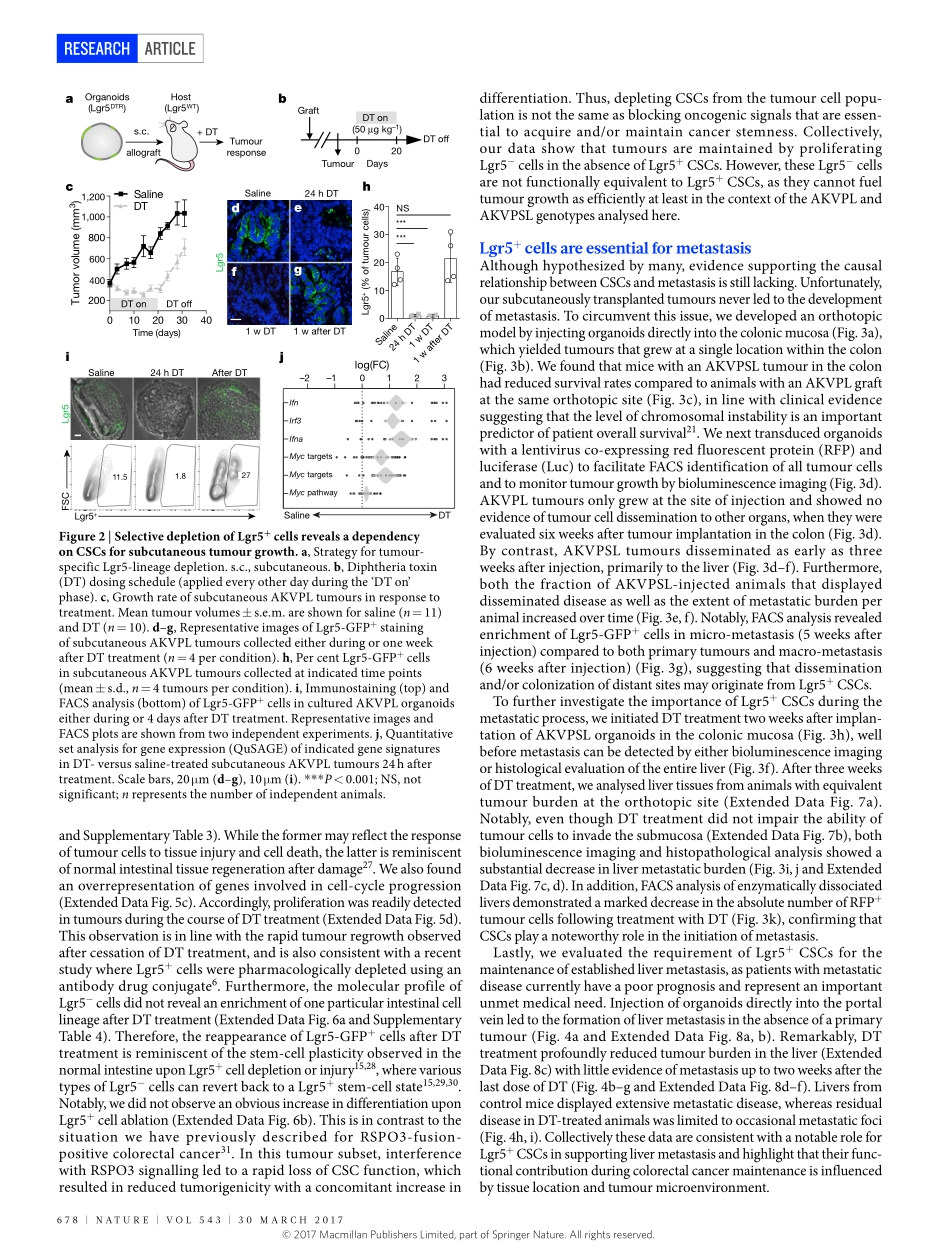
676|NATURE|VOL543|30MARCH2017ARTICLEdoi:10.1038/nature21713AdistinctroleforLgr5+stemcellsinprimaryandmetastaticcoloncancerFelipedeSousaeMelo1,AntoninaV.Kurtova1,JonathanM.Harnoss2,NoelynKljavin1,JoergD.Hoeck1,JeffreyHung3,JeffreyEasthamAnderson3,ElaineE.Storm1,ZoraModrusan4,HartmutKoeppen3,GerritJ.P.Dijkgraaf1,RobertPiskol5&FredericJ.deSauvage1Colorectalcancerisaleadingcauseofcancer-relateddeath1andprogressesowingtothesequentialacquisitionofspecificgeneticalter-ations,includingfunctionallossoftumoursuppressorssuchasAPC,TP53andSMAD4,aswellasactivatingmutationsinoncogenessuchasKRAS2.Onlyasubsetofcellswithinthesetumourshastumori-genicpotential3,4,suggestingahierarchicalorganization.Thesecells,referredtoasCSCs,arethoughttorepresenttheclonogeniccoreofthetumourandcanbeidentifiedbyspecificmarkers,includingleucine-richrepeat-containingG-protein-coupledreceptor5(LGR5)5–8.TargetingCSCsispredictedtohavewidespreadclinicalimplications,sinceitisassumedthatthesecellsareinvolvedintumourprogressionandmetastaticdissemination9.AlthoughCSCshavebeenidentifiedinvarioustumourtypes10,11,theirclinicalrelevanceremainsunclearandstillawaitsthedevelopmentofrelevantpre-clinicalexperimentalsystemsinwhichtheselectivedepletionofCSCscanbeachieved12,13.ModellingcolorectalcancerprogressionToinvestigatethefunctionalroleofCSCsincolorectalcancerinitiation,growthandmetastasis,wecombinedtheApcmin/+;KrasLSL-G12D/+;Vil1Cre(AKV)modelofintestinaltumorigenesis6,14withtheLgr5DTR/eGFPallele15.TheresultingApcmin/+;KrasLSL-G12D/+;Vil1Cre;Lgr5DTR/eGFP(AKVL)animals(Fig.1a)carrytwoofthemostfrequentlymutatedgenesfoundinhumancolorectalcancerand,inaddition,expressadiphtheriatoxinreceptor(DTR)fusedtoanenhancedgreenfluores-centprotein(eGFP)undertheendogenousregulatoryregionofLgr5.Asaresult,Lgr5+cellscanbevisualizedandablatedselectivelyafteradministrationofdiphtheriatoxin(DT).AlthoughtheseanimalsformpolypsthatcontainLgr5+cells,theanimalsarenotsuitabletostudythecontributionofthesecellstotumourgrowthbecauselong-termDTtrea...