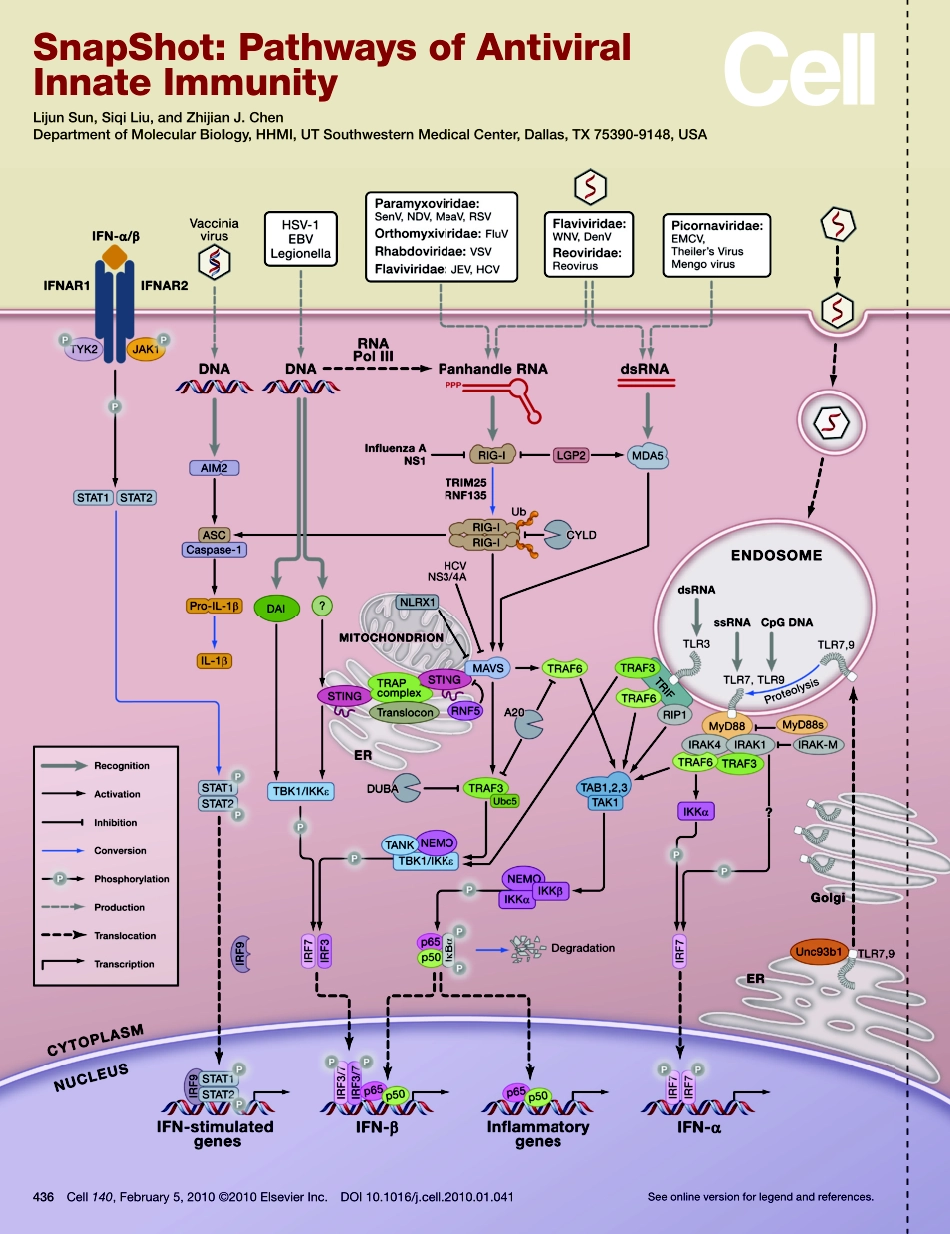
436Cell140,February5,2010©2010ElsevierInc.DOI10.1016/j.cell.2010.01.041Seeonlineversionforlegendandreferences.SnapShot:PathwaysofAntiviralInnateImmunityLijunSun,SiqiLiu,andZhijianJ.ChenDepartmentofMolecularBiology,HHMI,UTSouthwesternMedicalCenter,Dallas,TX75390-9148,USASnapShot:PathwaysofAntiviralInnateImmunityLijunSun,SiqiLiu,andZhijianJ.ChenDepartmentofMolecularBiology,HHMI,UTSouthwesternMedicalCenter,Dallas,TX75390-9148,USA436.e1Cell140,February5,2010©2010ElsevierInc.DOI10.1016/j.cell.2010.01.041Viraldiseasesremainachallengingglobalhealthissue.Innateimmunityisthefirstlineofdefenseagainstviralinfection.Ahallmarkofantiviralinnateimmuneresponsesistheproductionoftype1interferonsandinflammatorycytokines.Thesemoleculesnotonlyrapidlycontainviralinfectionbyinhibitingviralreplicationandassemblybutalsoplayacrucialroleinactivatingtheadaptiveimmunesystemtoeradicatethevirus.Recentresearchhasunveiledmultiplesignalingpathwaysthatdetectviralinfection,withseveralpathwaysdetectingthepresenceofviralnucleicacids.ThisSnapShotfocusesoninnatesignalingpathwaystriggeredbyviralnucleicacidsthataredeliveredtothecytosolandendosomesofmammalianhostcells.CytosolicPathwaysManyviralinfections,especiallythoseofRNAviruses,resultinthedeliveryandreplicationofviralRNAinthecytosolofinfectedhostcells.TheseviralRNAsoftencontain5′-triphosphate(5′-ppp)andpanhandle-likesecondarystructurescomposedofdouble-strandedsegments.ThesefeaturesarerecognizedbymembersoftheRIG-I-likeRecep-tor(RLR)family,whichincludesRIG-I,MDA5,andLGP2(Fujita,2009;Yoneyamaetal.,2004).AllRLRscontainaDEAD/H-boxRNAhelicasedomaininthemiddle.Inaddition,RIG-IandLGP2containaC-terminalregulatorydomain(RD)thatbindsto5′-pppRNA.MDA5,ontheotherhand,recognizeslongdouble-strandedRNAs(dsRNAs)aswellassingle-strandedRNAs(ssRNAs)derivedfrompicornaviruses.RIG-IandMDA5,butnotLGP2,alsocontaintwoN-terminalCARDdomainsthatinteractwiththeCARDdomainoftheadaptorproteinMAVS(alsoknownasIPS-1,VISA,orCARDIF),whichresidesinthemitochondrialoutermembrane.MAVSinte...