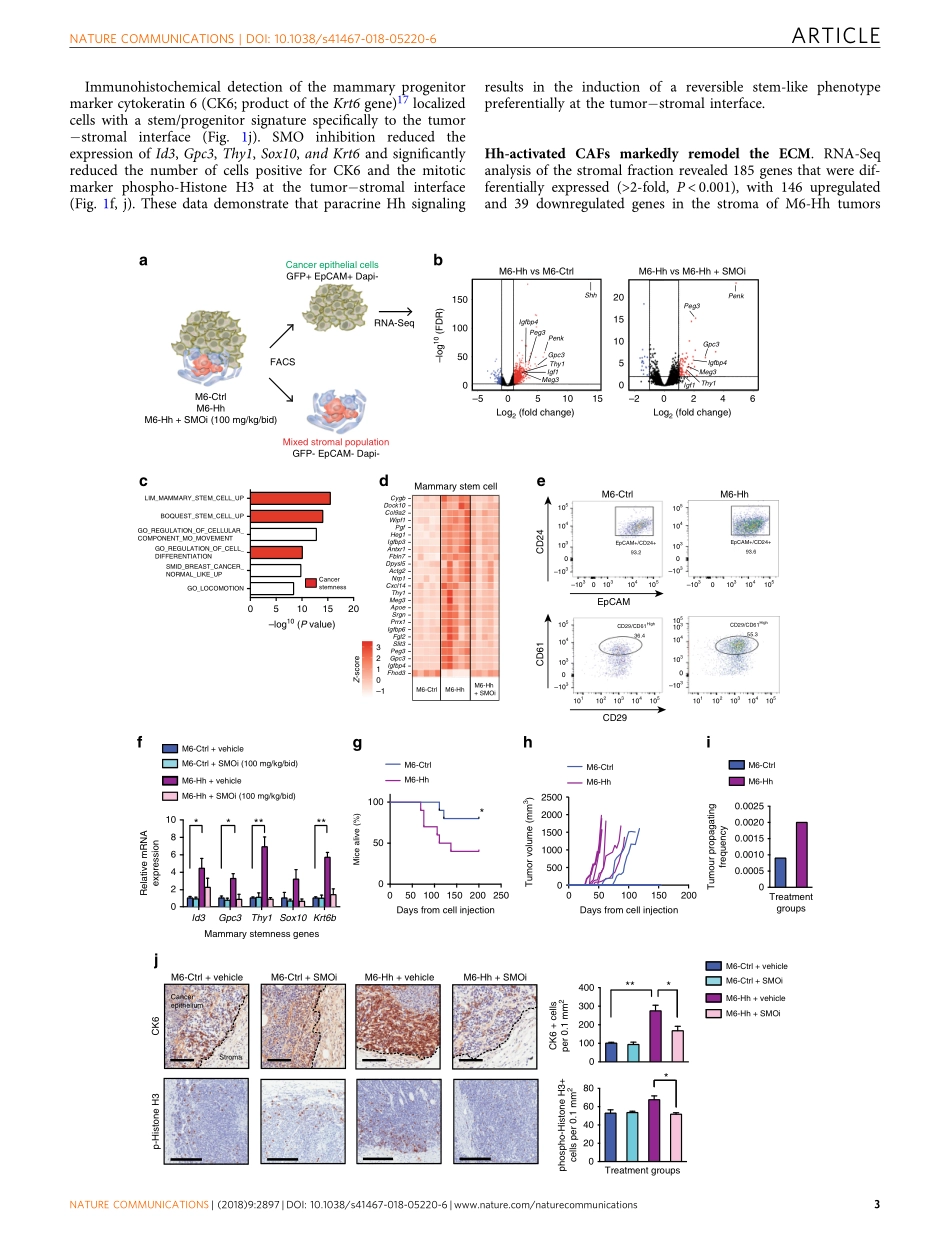
ARTICLETargetingstromalremodelingandcancerstemcellplasticityovercomeschemoresistanceintriplenegativebreastcancerAurélieS.Cazet1,2,3,MunN.Hui1,2,4,5,BenjaminL.Elsworth6,SunnyZ.Wu1,2,3,DanielRoden1,2,3,Chia-LingChan1,2,JoannaN.Skhinas1,2,RaphaëlCollot1,2,JessicaYang1,2,KateHarvey1,2,M.ZahiedJohan7,8,CarolineCooper9,RadhikaNair10,DavidHerrmann1,2,3,AndreaMcFarland1,2,NiantaoDeng1,2,3,ManuelRuiz-Borrego11,FedericoRojo12,JoséM.Trigo13,SusanaBezares14,RosalíaCaballero14,ElgeneLim1,2,3,15,PaulTimpson1,2,3,SandraO’Toole1,2,5,D.NeilWatkins1,2,3,15,ThomasR.Cox1,2,3,MichaelS.Samuel7,8,MiguelMartín16&AlexanderSwarbrick1,2,3Thecellularandmolecularbasisofstromalcellrecruitment,activationandcrosstalkincarcinomasispoorlyunderstood,limitingthedevelopmentoftargetedanti-stromaltherapies.Inmousemodelsoftriplenegativebreastcancer(TNBC),Hedgehogligandproducedbyneoplasticcellsreprogramscancer-associatedfibroblasts(CAFs)toprovideasupportivenichefortheacquisitionofachemo-resistant,cancerstemcell(CSC)phenotypeviaFGF5expressionandproductionoffibrillarcollagen.Stromaltreatmentofpatient-derivedxeno-graftswithsmoothenedinhibitors(SMOi)downregulatesCSCmarkersexpressionandsensitizestumorstodocetaxel,leadingtomarkedlyimprovedsurvivalandreducedmetastaticburden.InthephaseIclinicaltrialEDALINE,3of12patientswithmetastaticTNBCderivedclinicalbenefitfromcombinationtherapywiththeSMOiSonidegibanddocetaxelche-motherapy,withonepatientexperiencingacompleteresponse.ThesestudiesidentifyHedgehogsignalingtoCAFsasanovelmediatorofCSCplasticityandanexcitingnewtherapeutictargetinTNBC.DOI:10.1038/s41467-018-05220-6OPEN1GarvanInstituteofMedicalResearch,Darlinghurst,NSW2010,Australia.2TheKinghornCancerCentre,Darlinghurst,NSW2010,Australia.3StVincent’sClinicalSchool,FacultyofMedicine,UNSWSydney,Darlinghurst,NSW2010,Australia.4TheChrisO’BrienLifehouse,Camperdown,NSW2050,Australia.5RoyalPrinceAlfredHospital,Camperdown,NSW2050,Australia.6MRCIntegrativeEpidemiologyUnit,UniversityofBristol,OakfieldHouse,BristolBS82B...