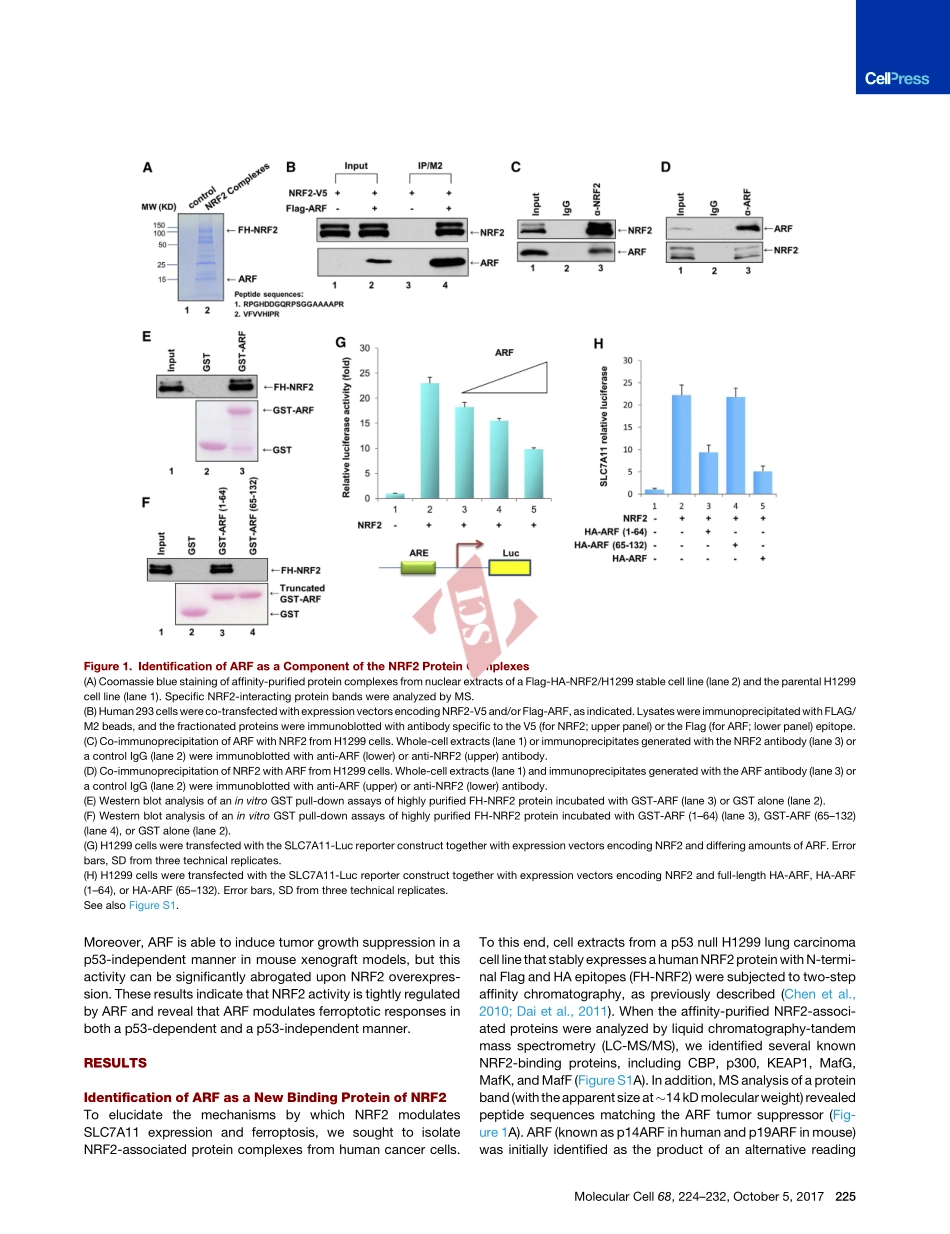
ShortArticleNRF2IsaMajorTargetofARFinp53-IndependentTumorSuppressionGraphicalAbstractHighlightsdARFinteractswithNRF2bothinvitroandinvivodARFinhibitstheabilityofNRF2toactivateitstargetgenesdTheARF-NRF2interactioniscriticalforferroptosisinp53nullcellsdNRF2isamajortargetforp53-independenttumorsuppressionbyARFAuthorsDelinChen,OmidTavana,BoChu,LukeErber,YueChen,RichardBaer,WeiGuCorrespondencewg8@cumc.columbia.eduInBriefChenetal.identifiedARFasakeyregulatorofNRF2-mediatedactivationofSLC7A11,acomponentofthecystine/glutamateantiporterthatregulatesreactiveoxygenspecies(ROS)-inducedferroptosis.TheyshowedthattheARF-NRF2interactioniscriticalforp53-independentferroptosisandtumorsuppressioninducedbyARF.Chenetal.,2017,MolecularCell68,224–232October5,2017ª2017ElsevierInc.http://dx.doi.org/10.1016/j.molcel.2017.09.009MolecularCellShortArticleNRF2IsaMajorTargetofARFinp53-IndependentTumorSuppressionDelinChen,1,2OmidTavana,1,2BoChu,1,2LukeErber,3YueChen,3RichardBaer,1,2andWeiGu1,2,4,*1InstituteforCancerGenetics,DepartmentofPathologyandCellBiology2HerbertIrvingComprehensiveCancerCenterCollegeofPhysiciansandSurgeons,ColumbiaUniversity,1130St.NicholasAvenue,NewYork,NY10032,USA3DepartmentsofBiochemistry,MolecularBiology,andBiophysics,UniversityofMinnesota,Minneapolis,MN55455,USA4LeadContact*Correspondence:wg8@cumc.columbia.eduhttp://dx.doi.org/10.1016/j.molcel.2017.09.009SUMMARYAlthoughARFcansuppresstumorgrowthbyacti-vatingp53function,themechanismsbywhichitsup-pressestumorgrowthindependentlyofp53arenotwellunderstood.Here,weidentifiedARFasakeyregulatorofnuclearfactorE2-relatedfactor2(NRF2)throughcomplexpurification.ARFinhibitstheabilityofNRF2totranscriptionallyactivateitstargetgenes,includingSLC7A11,acomponentofthecystine/glutamateantiporterthatregulatesreactiveoxygenspecies(ROS)-inducedferroptosis.Asaconse-quence,ARFexpressionsensitizescellstoferropto-sisinap53-independentmannerwhileARFdepletioninducesNRF2activationandpromotescancercellsurvivalinresponsetooxidativestress.Moreover,thea...