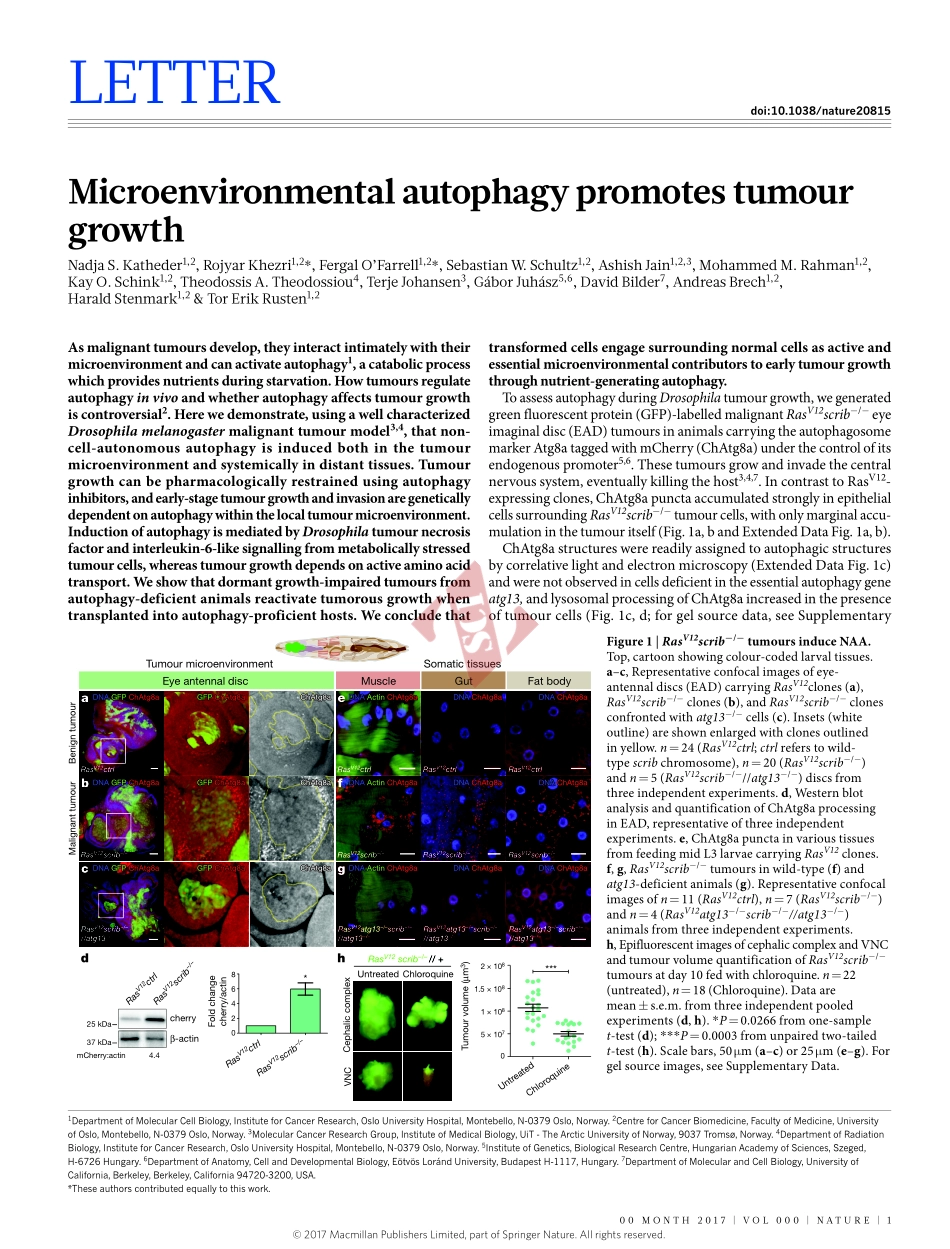
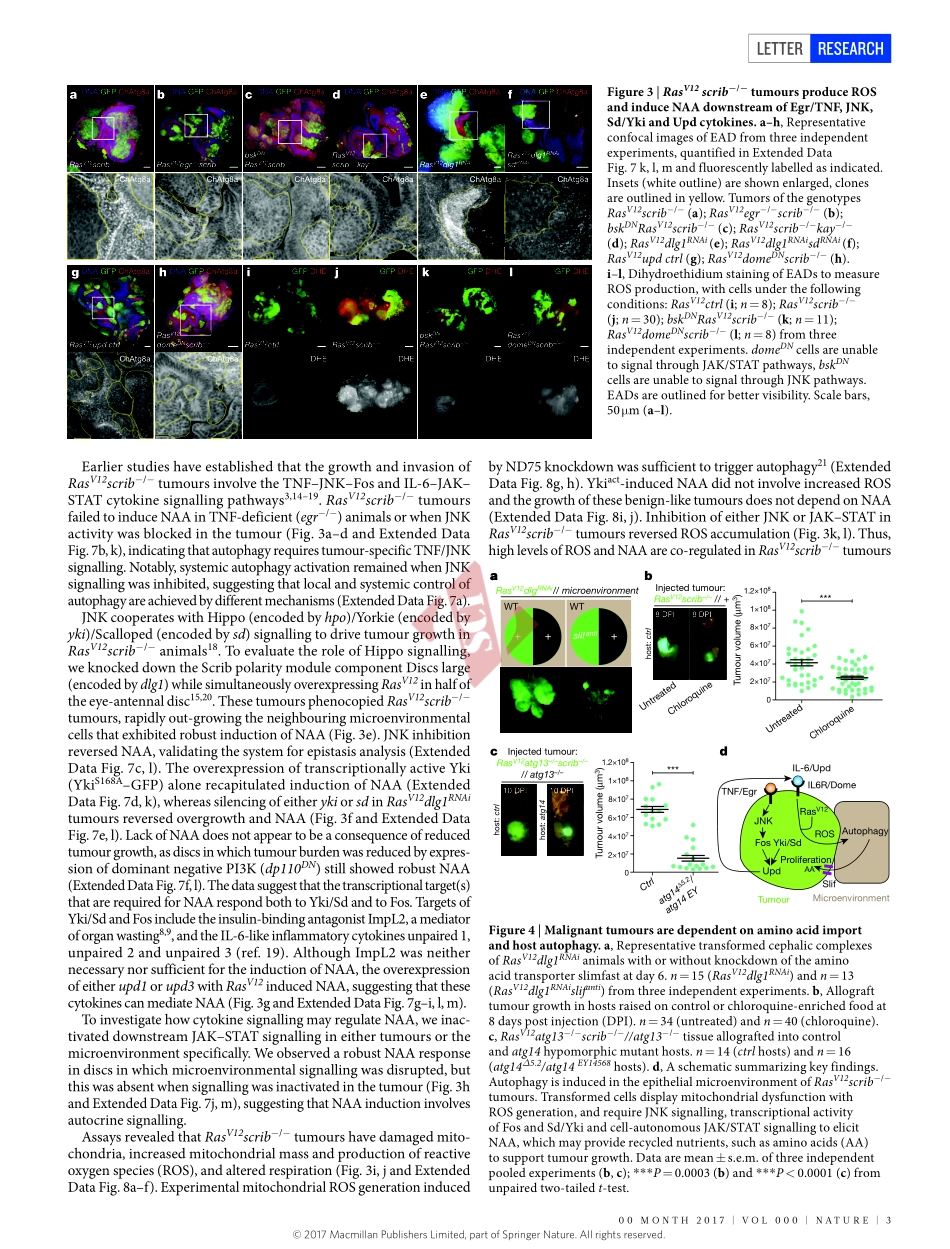
00Month2017|VoL000|nAtURE|1LEttERdoi:10.1038/nature20815MicroenvironmentalautophagypromotestumourgrowthnadjaS.Katheder1,2,RojyarKhezri1,2*,Fergalo’Farrell1,2*,SebastianW.Schultz1,2,AshishJain1,2,3,MohammedM.Rahman1,2,Kayo.Schink1,2,theodossisA.theodossiou4,terjeJohansen3,GáborJuhász5,6,DavidBilder7,AndreasBrech1,2,haraldStenmark1,2&torErikRusten1,2Asmalignanttumoursdevelop,theyinteractintimatelywiththeirmicroenvironmentandcanactivateautophagy1,acatabolicprocesswhichprovidesnutrientsduringstarvation.Howtumoursregulateautophagyinvivoandwhetherautophagyaffectstumourgrowthiscontroversial2.Herewedemonstrate,usingawellcharacterizedDrosophilamelanogastermalignanttumourmodel3,4,thatnon-cell-autonomousautophagyisinducedbothinthetumourmicroenvironmentandsystemicallyindistanttissues.Tumourgrowthcanbepharmacologicallyrestrainedusingautophagyinhibitors,andearly-stagetumourgrowthandinvasionaregeneticallydependentonautophagywithinthelocaltumourmicroenvironment.InductionofautophagyismediatedbyDrosophilatumournecrosisfactorandinterleukin-6-likesignallingfrommetabolicallystressedtumourcells,whereastumourgrowthdependsonactiveaminoacidtransport.Weshowthatdormantgrowth-impairedtumoursfromautophagy-deficientanimalsreactivatetumorousgrowthwhentransplantedintoautophagy-proficienthosts.Weconcludethattransformedcellsengagesurroundingnormalcellsasactiveandessentialmicroenvironmentalcontributorstoearlytumourgrowththroughnutrient-generatingautophagy.ToassessautophagyduringDrosophilatumourgrowth,wegeneratedgreenfluorescentprotein(GFP)-labelledmalignantRasV12scrib−/−eyeimaginaldisc(EAD)tumoursinanimalscarryingtheautophagosomemarkerAtg8ataggedwithmCherry(ChAtg8a)underthecontrolofitsendogenouspromoter5,6.Thesetumoursgrowandinvadethecentralnervoussystem,eventuallykillingthehost3,4,7.IncontrasttoRasV12-expressingclones,ChAtg8apunctaaccumulatedstronglyinepithelialcellssurroundingRasV12scrib−/−tumourcells,withonlymarginalaccu-mulationinthetumouritself(Fig.1a,bandExtendedDataFig.1a,b).ChAtg8as...