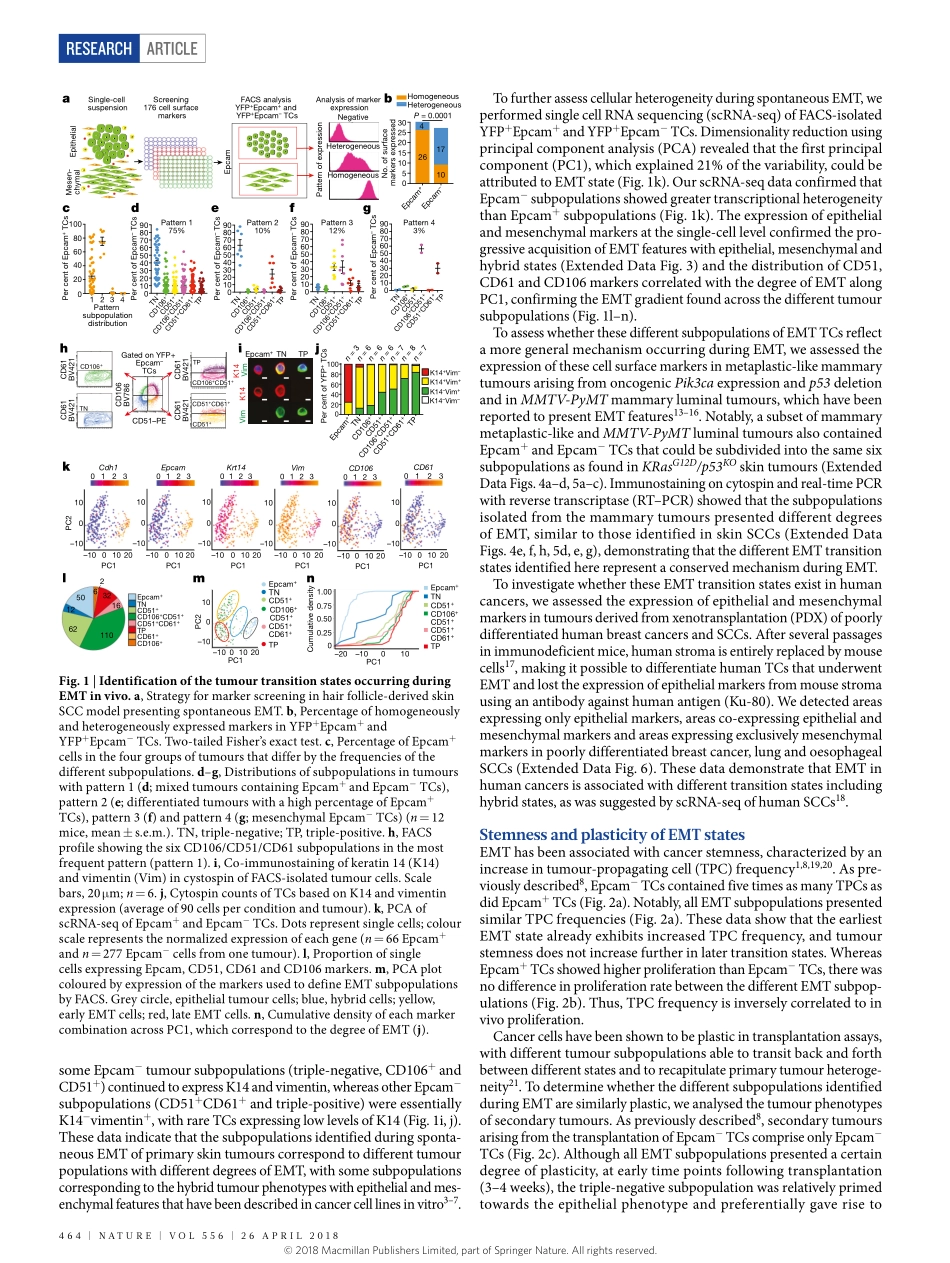
Articlehttps://doi.org/10.1038/s41586-018-0040-3IdentificationofthetumourtransitionstatesoccurringduringEMTievgeniaPastushenko1,AudreyBrisebarre1,AlejandroSifrim2,3,MarcoFioramonti1,tatianarevenco1,SoufianeBoumahdi1,AlexandraVanKeymeulen1,DanielBrown2,4,VirginieMoers1,Sophielemaire1,SarahDeclercq5,esmeraldaMinguijón5,cédricBalsat6,YouriSokolow7,christineDubois1,FlorianDecock1,SamuelScozzaro1,FedericoSopena8,Angellanas9,NickyD’Haene5,isabelleSalmon5,6,Jean-christopheMarine4,10,thierryVoet2,3,PanagiotaA.Sotiropoulou1,12&cédricBlanpain1,11,12*Incancer,theepithelial-to-mesenchymaltransition(EMT)isassociatedwithtumourstemness,metastasisandresistancetotherapy.Ithasrecentlybeenproposedthat,ratherthanbeingabinaryprocess,EMToccursthroughdistinctintermediatestates.However,thereisnodirectinvivoevidenceforthisidea.Herewescreenalargepanelofcellsurfacemarkersinskinandmammaryprimarytumours,andidentifytheexistenceofmultipletumoursubpopulationsassociatedwithdifferentEMTstages:fromepithelialtocompletelymesenchymalstates,passingthroughintermediatehybridstates.AlthoughallEMTsubpopulationspresentedsimilartumour-propagatingcellcapacity,theydisplayeddifferencesincellularplasticity,invasivenessandmetastaticpotential.Theirtranscriptionalandepigeneticlandscapesidentifytheunderlyinggeneregulatorynetworks,transcriptionfactorsandsignallingpathwaysthatcontrolthesedifferentEMTtransitionstates.Finally,thesetumoursubpopulationsarelocalizedindifferentnichesthatdifferentiallyregulateEMTtransitionstates.EMTisacellularprocessinwhichcellslosetheirepithelialcharacteris-ticsandacquiremesenchymalfeatures,whichenablethemtomigratemoreefficientlyandinvadetheunderlyingmesenchyme.EMTisessentialforgastrulation,somitogenesisandneuralcrestdelaminationduringembryonicdevelopmentandhasbeenassociatedwithvariousdiseases.Incancer,EMTisassociatedwithtumorigenesis,invasion,metastasis,tumourstemnessandresistancetotherapy1,2.AlthoughEMThastraditionallybeenviewedasabinaryswitch,someinvitrodata(mainlyco-expressionofepi...