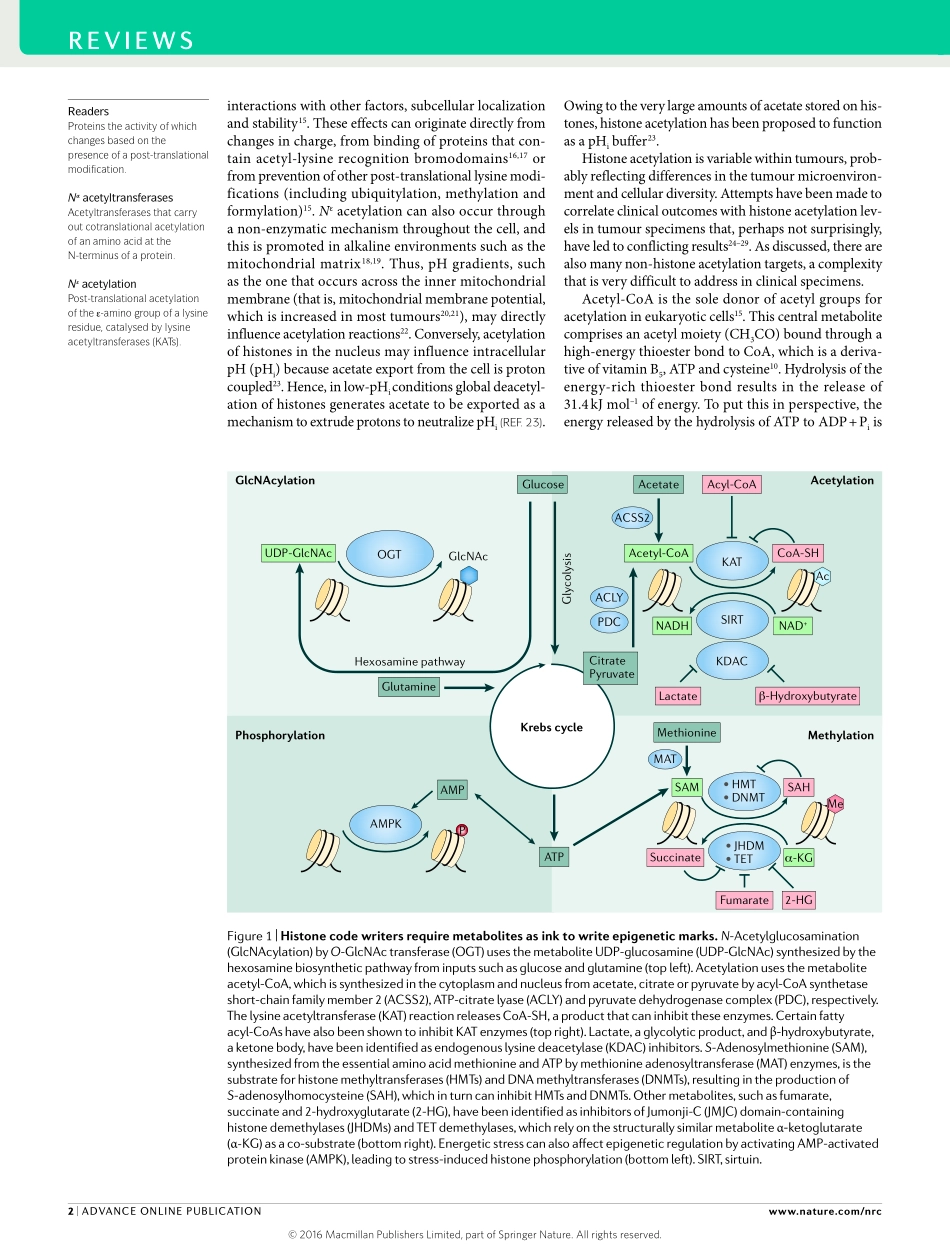
Epigeneticplasticityincancerfacilitatestheacquisitionofitshallmarkcharacteristics1,2.Themetabolictraitsoftumourcellsarealsocrucialforadjustingtochangesintheavailabilityofoxygenandnutrients(carbohydrates,lipidsandaminoacids)inthetumourmicroenvironment,tosustainproliferationandresistmitochondria-dependentapoptosis3–5.Cellularmetabolismandtheepigenomeinteractwithoneanotherandwiththegeneticandmoleculardriversofcancer,inabidirectionalmanner.Anintegrativeunderstandingoftheinterplaybetweenthemolecular,metabolicandepigeneticrewiringincancerisfarfromcomplete,butconceptualthemesarebeginningtoemerge.Furtherelucidationoftheselinksislikelytoleadtomoreeffectivecancertherapies.Mostpost-translationalmodifications(PTMs),suchasphosphorylation,acetylationandotheracylmodifi-cations,methylationandO-linkedN-acetylglucosaminemodification(O-GlcNAcylation),requiremetabolitesassubstrates(FIG.1).Inthenucleus,thesemetabolitesareusedforchromatinmodifications,includingacetyl-CoAforhistoneacetylationandS-adenosylmethionine(SAM)forhistoneandDNAmethylation.Thehistonecodehypothesisisbasedonwriters,erasersandreadersofchro-matinmarks6.Thisassumesthatthe‘ink’inthisprocessisneverlimiting;however,basedonagrowingbodyofevi-dencethattheavailabilityofmetabolitestothewritershasanimpactonchromatinmodifications,webelievethatitmaybetimetoaddafourthparameterinthiscode:themetabolite-producingenzymes,whichprovidetheinkforhistonemodification(FIG.1).InthisReview,wediscusshowmetaboliccontroloftheepigenomeisemergingasacrucialmechanismbywhichcancercellscanadapttoachangingenvironment.BasicbiochemistryofacetylationMorethan8,000uniqueacetylationsitesinproteinshavebeendetectedinmammaliancells7–9.Withinthenucleus,histonescomprisethebulkofacetylationloci.Thechro-matinofmammaliancellscontainsatleast10billionpotentialacetylationsites,meaningthataglobalchangeinhistoneacetylationmayleadtoasubstantialreductionincellularornuclearacetyl-CoAlevels.Giventhehighamountsofenergystoredinamoleculeofacetyl-CoA,thismayrepresentapotent...